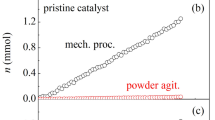
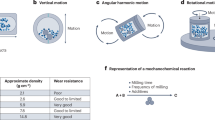
Abstract
THE thermodynamic functions ‘availability’ and ‘maximum work’, as they are usually defined1–3, are generally inapplicable to systems that interest the biologist and electrochemist for the reasons shown below. Here I propose the redefinition and generalisation of both functions, and explicitly define a thermodynamic property, the ‘mechanochemical availability’, that provides a measure of the maximum usable mechanochemical work that can be released in the interaction of a contractile system with a source or sink of chemical potential of constant intensive state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hatsopoulos, G. N., and Keenan, J. H., Principles of General Thermodynamics, 163 (Wiley, New York, 1965).
Wilkie, D. R., Nature, 245, 457–458 (1973).
Keenan, J., Thermodynamics (Wiley, New York, 1941).
Sussman, M., Elementary General Thermodynamics, 244–245 (Addison-Wesley, Reading, Massachusetts, 1972).
Zemansky, M., Heat and Thermodynamics, fourth ed., 168 (McGraw-Hill, New York, 1957).
Sussman, M., and Katchalsky, A., Science, 167, 47 (1970).
The collected works of J. Willard Gibbs, 1, 77 (Yale University Press, New Haven, 1948).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SUSSMAN, M. Mechanochemical availability. Nature 256, 195–198 (1975). https://doi.org/10.1038/256195a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/256195a0
This article is cited by
-
A polymer gel with electrically driven motility
Nature (1992)