Abstract
Study Design:
A basic study using a spinal cord injury (SCI) model in rats.
Objectives:
The effect of mild hypothermic treatment on histological changes and motor function after a rat spinal cord compression injury was assessed.
Methods:
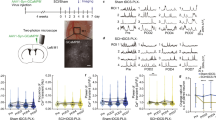
Mild spinal cord compression was performed at the eleventh thoracic vertebral level by a 20 g weight for 20 min. Rats in the mild hypothermic model were kept at a body temperature of 33 °C and rats in the normothermic group were kept at 37 °C for 1 h from beginning of compression. Motor function was evaluated by measuring the frequency of standing. Microglia were stained by isolectin B4 and observed in the compressed portion of the spinal cord. The amount of tumor necrosis factor-α (TNF-α) in the compressed spinal cord was measured by the ELISA method.
Results:
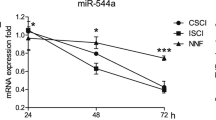
In the normothermic rats, microglia proliferated up to 72 h after the compression. Proliferation was substantially inhibited at 48 and 72 h after compression in the hypothermic rats. The motor function of the hypothermic rats improved at 48 and 72 h after the compression, whereas no improvement was seen in the normothermic rats. The amount of TNF-α in the compressed portion of the spinal cord was lower in hypothermic rats compared with normothermic rats throughout the experiment.
Conclusions:
These results suggest that hypothermic treatment is effective for the amelioration of delayed motor dysfunction via inhibition of microglial inflammatory responses.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kataoka K, Yanase H . Mild hypothermia—a revived countermeasure against ischemic neuronal damages. Neurosci Res 1998; 32: 103–117.
Bernard S . Induced hypothermia in intensive care medicine. Anaesth Intensive Care 1996; 24: 382–388.
Hayashi N, Hirayama T, Udagawa A, Daimon W, Ohata M . Systemic management of cerebral edema based on a new concept in severe head injury patients. Acta Neurochir Suppl 1994; 60: 541–543.
Morioka T, Kalehua AN, Streit WJ . The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab 1991; 11: 966–973.
Watanabe T, Yamamoto T, Abe Y, Saito N, Kumagai T, Kayama H . Differential activation of microglia after experimental spinal cord injury. J Neurotrauma 1999; 16: 255–265.
Meda L, Cassatella MA, Szendrei GI, Otvos Jr L, Baron P, Villalba M et al. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature 1995; 374: 647–650.
Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK . Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 1992; 149: 2736–2741.
Tanaka M, Sotomatsu A, Yoshida T . Detection of superoxide production by activated microglia using a sensitive and specific chemiluminescence assay and microglia-mediated PC 12 h cell death. J Neurochem 1994; 63: 266–270.
Morino T, Ogata T, Horiuchi H, Takeba J, Okumura H, Miyazaki T et al. Delayed neuronal damage related to microglia proliferation after mild spinal cord compression injury. Neurosci Res 2003; 46: 309–318.
Adelson PD, Ragheb J, Kanev P, Brockmeyer D, Beers SR, Brown SD et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery 2005; 56: 740–754.
Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD . Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987; 7: 729–738.
Lei MW, Ye Y, Liang JZ, Nai HJ, Zhi YXU . Moderate hypothermia prevents neural cell apoptosis following spinal cord ischemia in rabbits. Cell Res 2005; 15: 387–393.
Dietrich WD, Chatzipanteli K, Vitarbo E, Wada K, Kinoshita K . The role of inflammatory processes in the pathophysiology and treatment of brain and spinal cord trauma. Acta Neurochir Suppl 2004; 89: 69–74.
Luo J, Li N, Robinson JP, Shi R . The increase of reactive oxygen species and their inhibition in an isolated guinea pig spinal cord compression model. Spinal Cord 2002; 40: 656–665.
Burger R, Vince GH, Meixensberger J, Roosen K . Hypothermia influences time course of intracranial pressure, brain temperature, EEG and microcirculation during ischemia-reperfusion. Neurol Res 1998; 20: s52–s60.
Diretrich WD, Busto R, Halley M, Valdes I . The importance of brain temperature in alterations of the blood-brain barrier following cerebral ischemia. J Neuropathol Exp Neurol 1990; 49: 486–497.
Busto R, Dietrich WD, Globus MY, Ginsberg MD . Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neurosci Lett 1989; 101: 299–304.
Tsutsumi K, Ueda T, Shimizu H, Hashizume K, Yozu R . Effect of delayed induction of postischemic hypothermia on spinal cord damage induced by transient ischemic insult in rabbits. Jpn J Thorac Cardiovasc Surg 2004; 52: 411–418.
Westmoreland SV, Kolson D, Gonzalez-Scarano F . Toxicity of TNF alpha and platelet activating factor for human NT2N neurons: a tissue culture model for human immunodeficiency virus dementia. J Neurovirol 1996; 2: 118–126.
Zhang SC, Fedoroff S . Neuron-microglia interactions in vitro. Acta Neuropathol 1996; 91: 385–395.
Viviani B, Corsini E, Galli CL, Marinovich M . Glia increase degeneration of hippocampal neurons through release of tumor necrosisfactor-alpha. Toxicol Appl Pharmacol 1998; 150: 271–276.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morino, T., Ogata, T., Takeba, J. et al. Microglia inhibition is a target of mild hypothermic treatment after the spinal cord injury. Spinal Cord 46, 425–431 (2008). https://doi.org/10.1038/sj.sc.3102163
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.sc.3102163