Abstract
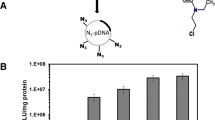
We investigated the efficacy and safety of the cationic polymer polyethylenimine (PEI) as a potential tool for intrauterine gene delivery into livers of fetal mice in the last trimester of pregnancy (E17.5). Using luciferase as a reporter gene, transferrin-conjugated and ligand-free PEI/DNA complexes (containing 3 μg DNA) with varying PEI-nitrogen/DNA-phosphate (N/P) ratios and different PEI forms, branched (800, 25 kDa) and linear (22 kDa), were compared with naked DNA. Transgene expression was measured 48 h after administration of PEI/DNA complexes or naked DNA. Highest luciferase activity (9.8 × 103 relative light units (RLU)/mg of tissue protein) was observed with ligand-free PEI22/DNA mixtures at N/P 6.0. In addition, this formulation was associated with very low toxicity as compared to the other PEI/DNA-injected groups. Using β-galactosidase as a reporter gene, transfection of single, but also small, clusters of cells was demonstrated throughout the liver. Injection of 3 μg naked DNA resulted in an 11-fold lower transgene expression value (0.9 × 103 RLU/mg of tissue protein) as compared to PEI22/DNA complexes. However, the administration of higher concentrated naked DNA (9 μg) into fetal livers yielded expression levels of 3.2 × 104 RLU/mg of tissue protein, a more than three-fold increase compared to PEI22/DNA complexes. Furthermore, the gene transfer efficacy of concentrated naked DNA was approximately 40 times higher in fetuses than in adults (0.8 × 103 RLU/mg of tissue protein), indicating that fetal tissue is especially amenable to the uptake and expression of naked DNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Holtzman DM et al. Nerve growth factor protects the neonatal brain against hypoxic–ischemic injury. Ann Neurol 1996; 39: 114–122.
Mason CAE et al. Gene transfer in utero biologically engineers a patent ductus arteriosus in lambs by arresting fibronectin-dependent neointimal formation. Nat Med 1999; 5, 2: 176–182.
Prenatal Gene Transfer: Scientific, Medical, and Ethical Issues. A Report of the Recombinant DNA Advisory Committee. Hum Gene Ther 2000; 11: 1211–1229.
Tarantal AF et al. Rhesus monkey model for fetal gene transfer: studies with retroviral-based vector systems. Mol Ther 2001; 3: 128–138.
Douar AM et al. Foetal gene delivery in mice by intra-amniotic administration of retroviral producer cells and adenovirus. Gene Ther 1997; 4: 883–890.
Turkay A, Saunders T, Kurachi K . Intrauterine gene transfer: gestational stage-specific gene delivery in mice. Gene Ther 1999; 6: 1685–1694.
Lipshutz GS et al. In utero delivery of adeno-associated viral vectors: intraperitoneal gene transfer produces long-term expression. Mol Ther 2001; 3: 284–292.
Yang Y et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA 1994; 91: 4407–4411.
Gordon JW . Germline alteration by gene therapy: assessing and reducing the risks. Mol Med Today 1998; 4: 468–470.
Tsukamoto M et al. Gene transfer and expression in progeny after intravenous DNA injection into pregnant mice. Nat Genet 1995; 9: 243–248.
Gaensler KM et al. Fetal gene transfer by transuterine injection of cationic liposome–DNA complexes. Nat Biotech 1999; 17: 1188–1192.
Boussif O et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 1995; 92: 7297–7301.
Chemin I et al. Liver-directed gene transfer: a linear polyethylenimine derivative mediates highly efficient DNA delivery to primary hepatocytes in vitro and in vivo. J Viral Hepatol 1998; 5: 369–375.
Kircheis R et al. Polycation-based DNA complexes for tumor-targeted gene delivery in vivo. J Gene Med 1999; 1: 111–120.
Kircheis R et al. Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application. Gene Ther 2001; 8: 28–40.
Wagner E et al. Coupling of adenovirus to tranferrin–polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci USA 1992; 89: 6099–6103.
Wagner E et al. Delivery of drugs, proteins and genes into cells using transferrin as a ligand for receptor-mediated endocytosis. Adv Drug Del Rev 1994; 14: 113–136.
Goula D et al. Polyethylenimine-based intravenous delivery of transgenes to mouse lungs. Gene Ther 1998; 5: 1291–1295.
Wightman L et al. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J Gene Med 2001; 3: 362–372.
Wolff JA et al. Direct gene transfer into mouse muscle in vivo. Science 1990; 247: 1465–1468.
Hickman MA et al. Gene expression following direct injection of DNA into liver. Hum Gene Ther 1994; 5: 1477–1483.
Hengge UR, Walker PS, Vogel JC . Expression of naked DNA in human, pig, and mouse skin. J Clin Invest 1996; 97: 2911–2916.
Liu F, Song Y, Liu D . Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 1999; 6: 1258–1266.
Fraser R et al. High perfusion pressure damages the sieving ability of sinusoidal endothelium in rat livers. Br J Exp Pathol 1980; 61: 222–228.
Takeshita S, Isshiki T, Souto T . Increased expression of direct gene transfer into skeletal muscles observed after acute ischemic injury in rats. Lab Invest 1996; 74: 1061–1065.
Minguell J, Salinas F, Perretta M . Nucleic acid metabolism in the embryonic rat liver. Growth 1969; 33: 217–220.
Kohler E . Activity of some enzymes of nucleic acid metabolism in developing rat liver. Naunyn Schmiedebergs Arch Pharmacol 1972; 274: 385–393.
Rossi R, Viola-Magni MP . Changes in endonuclease activity and in chromatin structure of rat hepatocytes during fetal and neonatal life. Cell Differ 1982; 11: 91–98.
Wattiaux R, Laurent N, Wattiaux-De Coninck S, Jadot M . Endosomes, lysosomes: their implication in gene transfer. Adv Drug Deliv Rev 2000; 41: 201–208.
Lechardeur D et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther 1999; 6: 482–497.
Wolff JA et al. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet 1992; 1: 363–369.
Manthorpe M et al. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum Gene Ther 1993; 4: 419–431.
Winegar RA et al. Determination of tissue distribution of an intramuscular plasmid vaccine using PCR and in situ DNA hybridization. Hum Gene Ther 1996; 7: 2185–2194.
Kircheis R et al. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther 1997; 4: 409–418.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 1976; 72: 248–254.
Lim K, Chae CB . A simple assay for DNA transfection by incubation of the cells in culture dishes with substrates for beta-galactosidase. BioTechniques 1989; 7: 576–579.
Acknowledgements
We thank Andrea Fuchsbichler for performing electron microscopy, Vanessa Rössler for technical help with luciferase measurement and Dr Charles Buck for critically reading the manuscript. This work was supported by grants from the Austrian Science Fund (S 7401- MOB to KZ) and the Austrian Federal Ministry for Social Security and Generations (to KZ).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gharwan, H., Wightman, L., Kircheis, R. et al. Nonviral gene transfer into fetal mouse livers (a comparison between the cationic polymer PEI and naked DNA). Gene Ther 10, 810–817 (2003). https://doi.org/10.1038/sj.gt.3301954
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.gt.3301954
Keywords
This article is cited by
-
Transgene therapy for rat anti-Thy1.1 glomerulonephritis via mesangial cell vector with a polyethylenimine/decorin nanocomplex
Nanoscale Research Letters (2012)
-
Targeted polymeric gene delivery for anti-angiogenic tumor therapy
Macromolecular Research (2007)