Abstract
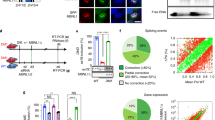
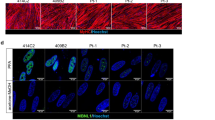
Myotonic dystrophy (DM1) is caused by the expansion of a trinucleotide repeat (CTG) located in the 3′untranslated region of the myotonic dystrophy protein kinase gene, for which currently there is no effective treatment. The data available suggest that misregulation of RNA homeostasis may play a major role in DM1 muscle pathogenesis. This indicates that the specific targeting of the mutant DMPK transcripts is essential to raise the rationale basis for the development of a specific gene therapy for DM1. We have produced a retrovirus which expresses a 149-bp antisense RNA complementary to the (CUG)13 repeats and to the 110-bp region following the repeats sequence to increase the specificity. This construct was introduced into human DM1 myoblasts, resulting in a preferential decrease in mutant DMPK transcripts, and effective restoration of human DM1 myoblast functions such as myoblast fusion and the uptake of glucose. It was previously shown that delay of muscle differentiation and insulin resistance in DM1 are associated with misregulation of CUGBP1 protein levels. The analysis of CUGBP1 levels and activity in DM1 cells expressing the antisense RNA indicated a correction of CUGBP1 expression in infected DM1 cells. We therefore show that current antisense RNA delivered in vitro using a retrovirus is not only capable of inhibiting mutant DMPK transcripts, but also can ameliorate dystrophic muscle pathology at the cellular levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Harper PS . Myotonic Dystrophy, 3rd edn. W.B. Saunder: London, UK, 1989, pp 293–322.
Brooke JD et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell 1992; 68: 799–808.
Fu YH et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 1992; 255: 1256–1258.
Mahadevan M et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science 1992; 255: 1253–1255.
Fu YH et al. Decreased expression of myotonin–protein kinase mRNA and protein in adult form of myotonic dystrophy. Science 1993; 260: 235–238.
Novelli G et al. Failure in detecting mRNA transcripts from the mutated allele in myotonic dystrophy muscle. Biochem Mol Biol Int 1993; 29: 291–297.
Hofmann-Radvanyi H et al. Myotonic dystrophy: absence of CTG enlarged transcript in congenital forms, and low expression of the normal allele. Hum Mol Genet 1993; 2: 1263–1267.
Boucher CA et al. A novel homeodomain-encoding gene is associated with a large CpG island interrupted by the myotonic dystrophy unstable (CTG)n repeat. Hum Mol Genet 1995; 4: 1919–1925.
Jansen G et al. Structural organization and development expression pattern of the DMR-N9 gene immediately upstream of the myotonic dystrophy locus. Hum Mol Genet 1995; 4: 843–852.
Davis MB et al. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc Natl Acad Sci 1997; 94: 7388–7393.
Timchenko LT et al. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucl Acid Res 1996; 24: 4407–4414.
Roberts R et al. Altered phosphorylation and intracellular distribution of a (CUG)(n) triplet repeat RNA binding protein in patients with myotonic dystrophy and in myotonin protein kinase knockout mice. Proc Natl Acad Sci 1997; 94: 13221–13226.
Philips AV, Timchenko LT, Cooper TA . Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 1998; 280: 737–741.
Timchenko NA et al. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J Biol Chem 2001; 276: 7820–7826.
Tiscornia G, Mahadevan MS . Myotonic dystrophy: the role of the CUG triplet repeats in splicing of a novel DMPK exon and altered cytoplasmic DMPK mRNA isoform ratios. Mol Cell 2000; 5: 959–967.
Miller JW et al. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J 2000; 19: 4439–4448.
Mankodi A et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 2000; 289: 1769–1773.
Weiss B, Davidkova G, Zhou LW . Antisense RNA gene therapy for studying and moduling biological processes. Cell Mol Life Sci 1999; 55: 334–358.
Weiss B . Antisense Oligonucleotides and Antisense RNA: Novel Pharmacological and Therapeutic Agents. CRC Press: Boca Raton, FL, 1997.
Birikh KR, Heaton PA, Eckstein F . The structure, function and application of the hammerhead ribozyme. Eur J Biochem 1997; 245: 1–16.
Caplen NJ et al. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci USA 2001; 98: 9742–9747.
Bass BL, Weintraub H . An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell 1988; 55: 1089–1098.
Scadden AD, Smith CW . Specific cleavage of hyper-edited dsRNAs. EMBO J 2001; 20: 4243–4252.
Deschenes I et al. Increase in the proliferative capacity of human myoblasts by using the T antigen under the vimentin promoter control. Muscle & Nerve 1997; 20: 437–445.
Furling D, Lemieux D, Taneja K, Puymirat J . Decreased levels of myotonic dystrophy protein kinase (DMPK) and delayed differentiation in human myotonic dystrophy myoblasts. Neuromuscul Disord 2001; 11: 728–735.
Furling D, Marette A, Puymirat J . Insulin-like growth factor-I circumvents defective insulin action in human myotonic dystrophy skeletal muscle cells. Endocrinology 1999; 140: 4244–4250.
Samuel CE . Mechanism of interferon action. Kinetics of interferon action in mouse L929 cells: phosphorylation of protein synthesis initiation factor elF-2 and ribosome-associated protein P1. Virology 1979; 93: 281–285.
Samuel CE . Protein–nucleic acid interactions and cellular responses to interferon. Methods 1998; 15: 161–165.
Zhou A, Hassel BA, Silverman RH . Expression cloning of 2-5A-dependent RNase: a uniquely regulated mediator of interferon action. Cell 1993; 72: 753–765.
Silverman RH . 2-5A dependent RNase L: a regulated endoribonuclease in the interferon system. In: D'Alessio G, Riodan JF (eds). Ribonucleases: Structure and Functions. Academic Press: New York, NY, 1997; pp 515–551.
Davis MB et al. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc Natl Acad Sci 1997; 94: 7388–7393.
Furling D et al. Defective satellite cells in congenital myotonic dystrophy. Hum Mol Genet 2001; 10: 2079–2087.
Timchenko NA et al. Molecular basis for impaired muscle differentiation in myotonic dystrophy. Mol Cell Biol 2001; 2: 6927–6938.
Savkur RS, Philips AV, Cooper TA . Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet 2001; 29: 40–47.
Denizot F, Lang R . Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986; 89: 271–277.
Sarabia V et al. Glucose transport in human skeletal muscle cells in culture. Stimulation by insulin and metformin. J Clin Invest 1992; 90: 1386–1395.
Acknowledgements
This work was supported by MDA (USA) grants and by grants from the Institutes of Health Research of Canada (IRSC), National Institutes of Health (AR01D44387 and AG16392) and the French Myopathy Association (AFM). We thank Dr Mahadevan for PUC18 5′UTR DMPK plasmid.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Furling, D., Doucet, G., Langlois, MA. et al. Viral vector producing antisense RNA restores myotonic dystrophy myoblast functions. Gene Ther 10, 795–802 (2003). https://doi.org/10.1038/sj.gt.3301955
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.gt.3301955
Keywords
This article is cited by
-
miR-322/-503 rescues myoblast defects in myotonic dystrophy type 1 cell model by targeting CUG repeats
Cell Death & Disease (2020)
-
Abnormal nuclear aggregation and myotube degeneration in myotonic dystrophy type 1
Neurological Sciences (2019)
-
Antisense oligonucleotides: the next frontier for treatment of neurological disorders
Nature Reviews Neurology (2018)
-
Human iPSC Models to Study Orphan Diseases: Muscular Dystrophies
Current Stem Cell Reports (2018)
-
Targeting RNA to treat neuromuscular disease
Nature Reviews Drug Discovery (2011)