Abstract
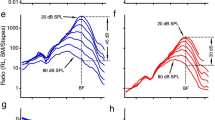
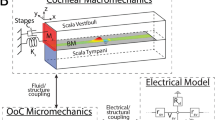
IT is thought that the sensitivity of mammalian hearing depends on amplification of the incoming sound within the cochlea by a select population of sensory cells, the outer hair cells. It has been suggested that these cells sense displacements and feedback forces which enhance the basilar membrane motion by reducing the inherent damping of the cochlear partition1–7. In support of this hypothesis, outer hair cells show membrane-potential-induced length changes1–3 at acoustic rates. This process has been termed 'reverse transduction'. For amplification, the forces should be large enough to move the basilar membrane. Using a displacement-sensitive interferometer8, we tested this hypothesis in an isolated cochlea while stimulating the outer hair cells with current passed across the partition. We show here that the cochlear partition distorts under the action of electrically driven hair cell length changes and produces place-specific vibration of the basilar membrane of a magnitude comparable to that observed near auditory threshold (about 1 nm). Such measurements supply direct evidence that cochlear amplification arises from the properties of the outer hair cell population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Brownell, W. E., Bader, C. R., Bertrand, D. & de Ripaubierre, Y. Science 227, 194–196 (1985).
Dallos, P., Evans, B. N. & Hallworth, R. Nature 350, 155–157 (1991).
Ashmore, J. F. J. Physiol. 388, 323–347 (1987).
Neely, S. T. & Kim, D. O. J. acoust. Soc. Am. 79, 1472–1480 (1986).
Dallos, P. in Auditory Function: Neurobiological Bases of Hearing (eds Edelman, G. M., Gall, W. E. & Cowan, W. M.) 153–188 (Wiley, New York, 1988).
Hubbard, A. Science 259, 68–71 (1993).
Davis, H. Hearing Res. 9, 79–90 (1983).
Mammano, F. & Ashmore, J. F. J. Physiol. 452, 169P (1992).
Dallos, P. Hearing Res. 14, 281–291 (1984).
Ashmore, J. F. & Meech, R. W. M. Nature, 322, 368–371 (1986).
Evans, B. N., Hallworth, R. & Dallos, P. Soc. Neurosci. Abstr. 14, 800 (1988).
Evans, E. F., Wilson, J. P. & Borerwe, T. A. ‘Tinnitus’, Ciba Foundation Symposium 85, 108–138 (1981).
Stypulkowsky, P. H. Hearing Res. 46, 113–143 (1990).
McFadden, D. & Plattsmier, H. S. J. acoust. Soc. Am. 76, 443–448 (1984).
Shehata, W. E., Brownell, W. E. & Dieler, R. Acta otolar. 111, 707–718 (1991).
Santos-Sacchi, J. J. Neurosci. 9, 2954–2962 (1989).
Santos-Sacchi, J. J. Neurosci. 11, 3096–3110 (1991).
Davis, H. Laryngoscope 68, 359–382 (1958).
Kolston, P. J., de Boer, E., Viergever, M. & Smoorenberg, G. J. acoust. Soc. Am. 86, 133–140 (1989).
Mammano, F. & Nobili, R. J. acoust. Soc. Am. 93, 3320–3332 (1993).
Greenwood, D. D. J. acoust. Soc. Am. 87, 2592–2605 (1990).
Ulfendahl, M., Khanna, S. M. & Flock, Å. Hearing Res. 40, 55–64 (1989).
Hubbard, A. E. & Mountain, D. C. Science 222, 510–512 (1983).
Reuter, G. & Zenner, H. P. Hearing Res. 43, 219–230 (1990).
Reuter, G., Gitter, A., Thurm, U. & Zenner, H. P. Hearing Res. 60, 236–246 (1992).
Sellick, P. M., Patuzzi, R. B. & Johnstone, B. M. J. acoust. Soc. Am. 72, 131–141 (1982).
Ruggero, M. & Rich, N. J. Neurosci. 11, 1057–1067 (1991).
Nuttall, A. L., Dolan, D. F. & Avinash, G. Hearing Res. 51, 203–214 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mammano, F., Ashmore, J. Reverse transduction measured in the isolated cochlea by laser Michelson interferometry. Nature 365, 838–841 (1993). https://doi.org/10.1038/365838a0
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/365838a0
This article is cited by
-
Intracochlear distortion products are broadly generated by outer hair cells but their contributions to otoacoustic emissions are spatially restricted
Scientific Reports (2021)
-
Vibration hotspots reveal longitudinal funneling of sound-evoked motion in the mammalian cochlea
Nature Communications (2018)
-
In vivo genetic manipulation of inner ear connexin expression by bovine adeno-associated viral vectors
Scientific Reports (2017)
-
Reverse transduction measured in the living cochlea by low-coherence heterodyne interferometry
Nature Communications (2016)
-
Scanning Electron Microscopic Examination of the Extracellular Matrix in the Decellularized Mouse and Human Cochlea
Journal of the Association for Research in Otolaryngology (2016)