Abstract
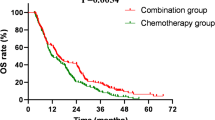
A randomized trial was conducted to determine whether administration of Amifostine with chemotherapy for small cell lung cancer could decrease the toxicity. 84 patients with small cell lung cancer of favourable prognosis (limited disease, performance status 0–1; limited disease with performance status 2 but normal sodium and alkaline phosphatase, or extensive diseas with performance status 0–1, normal sodium and alkaline phosphatase) received treatment with Ifosfamide 3 g/m2 intravenously, Carboplatin (Glomerular filtration rate + 25) × 6 mg intravenously, Etoposide 50 mg orally, twice daily, for 7 days, every 3 weeks. Patients were randomized to receive amifostine 740 mg/m2 immediately prior to the intravenous drugs (n = 42) or to receive chemotherapy alone (n = 42). The two groups were similar with respect to baseline prognostic factors. There was no significant difference in the occurrence of grade III or IV neutropenia or thrombocytopenia between the two groups, nor in the response rate or overall survival, for which the median was 11 months in the chemotherapy only group and 14 months in the group treated with amifostine. This study has not shown a protective effect from the use of amifostine with this regimen and there does not appear to be any effect upon the efficacy of treatment. © 2001 Cancer Research Campaign
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Glick J, Kemp G and Rose P (1992) A randomized trial of cyclophosphamide and cisplatin + WR-2721 in the treatment of advanced epithelial ovarian cancer. ASCO abstracts 11: 109
Glover D, Glick JH, Weiler C, Hurowitz S and Kligerman MM (1986) WR-2721 protects against the hematologic toxicity of cyclophosphamide: a controlled phase II trial. J Clin Oncol 4: 584–588
Kaplan EL and Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481
Miles DW, Fogarty O and Ash CM (1994) Received dose-intensity: a randomized trial of weekly chemotherapy with and without granulocyte colony-stimulating factor in small-cell lung cancer. J Clin Oncol 12: 77–82
Prendiville J, Lorigan P, Hicks F, Leahy B, Stout R, Burt P and Thatcher N (1994) Therapy for small cell lung cancer using carboplatin, ifosfamide, etoposide (without dose reduction), mid-cycle vincristine with thoracic and cranial irradiation. Eur J Cancer 14: 2085–2090
Roth BJ, Johnson DH and Einhorn LH (1992) Randomized study of cyclophosphamide plus doxorubicin plus vincristine versus etoposide plus cisplatin versus alternation of these two regimens in extensive small cell lung cancer: A phase III trial of the Southwestern Oncology Group. Journal of Clinical Oncology 10: 282–291
Shevlin PM, Muers MF, Peake MD, Hosker HS, Stead ML, Poulter KM and Brown JM (1998) Modified ice study: a phase II study of an intensive, modified ICE regimen (ifosfamide, carboplatin and etoposide) in patients with better prognosis, small cell lung cancer. Lung Cancer 21: 115–126
Souhami RL and Law K (1990) Longevity in small cell lung cancer. A report to the Lung Cancer Subcommittee of the United Kingdom Coordinating Committee for Cancer Research. Br J Cancer 61: 584–589
Steward WP, von Pawel J, Gatzemeier U, Woll P, Thatcher N, Koschel G, Clancy L, Verweij J, de Wit R, Pfeifer W, Fennelly J, von Eiff M and Frisch J (1998) Effects of granulocyte-macrophage colony-stimulating factor and dose intensification of V-ICE chemotherapy in small-cell lung cancer: a prospective randomized study of 300 patients. J Clin Oncol 16: 642–650
Thatcher N, Girling DJ, Hopwood P, Sambrook RJ, Qian W and Stephens RJ (2000) Improving survival without reducing quality of life in small-cell lung cancer patients by increasing the dose-intensity of chemotherapy with granulocyte colony-stimulating factor support: results of a British Medical Research Council Multicenter Randomized Trial. Medical Research Council Lung Cancer Working Party. J Clin Oncol 18: 395–404
van der Vijgh WJF and Peters GJ (1994) Protection of normal tissues from the cytotoxic effects of chemotherapy and radiation by amifostine (ethyol): Preclinical aspects. Seminars in Oncology 21: 2–7
WHO (1979). Handbook for reporting results of cancer treatments, WHO Offset Publication, No 48: Geneva
Yuhas JM (1980) Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Research 40: 1519–1524
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Johnson, P., Muers, M., Peake, M. et al. A randomized trial of amifostine as a cytoprotective agent in patients receiving chemotherapy for small cell lung cancer. Br J Cancer 84, 19–24 (2001). https://doi.org/10.1054/bjoc.2000.1539
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1054/bjoc.2000.1539