ABSTRACT
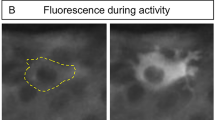
Spontaneous Ca2+ oscillations in vascular smooth muscle cells have been modeled using a single Ca2+ pool. This report describes spontaneous Ca2+ oscillations dependent on two separate Ca2+ sources for the nuclear versus cytoplasmic compartments. Changes in free intracellular Ca2+ were monitored with ratiometric Ca2+- fluorophores using confocal microscopy. On average, spontaneous oscillations developed in 79% of rat aortic smooth muscle cells that were synchronous between the cytoplasm and nucleus. Reduction of extracellular Ca2+ (< 1 μM) decreased the frequency and amplitude of the cytoplasmic oscillations with 48% of the oscillations asynchronous between the nuclear and cytoplasmic compartments. Similar results were obtained with the Ca2+ channel blockers, nimodipine and diltiazem. Arg-vasopressin (AVP) induced a rapid release of intracellular Ca2+ stores that was greater in the nuclear compartment (4.20 ± 0.23 ratio units, n = 56) than cytoplasm (2.54 ± 0.28) in cells that had spontaneously developed prior oscillations. Conversely, cells in the same conditions lacking oscillations had a greater AVP-induced Ca2+ transient in the cytoplasm (4.99 ± 0.66, n = 17) than in the nucleus (2.67 ± 0.29). Pre-treatment with Ca2+ channel blockers depressed the AVP responses in both compartments with the cytoplasmic Ca2+ most diminished. Depletion of internal Ca2+ stores prior to AVP exposure blunted the nuclear response, mimicking the response of cells that lacked prior oscillations. Spontaneous oscillating cells had a greater sarcoplasmic reticulum network than cells that did not oscillate. We propose that spontaneous nuclear oscillations rely on perinuclear sarcoplasmic reticulum stores, while the cytoplasmic oscillations rely on Ca2+ influx.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Pabelick CM, Sieck GC, Prakash YS . Invited review: significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J Appl Physiol 2001; 91(1):488–96.
Otun H, Gillespie JI, Nicholls JA, Greenwell JR, Dunlop W . Transients in intracellular free calcium in subconfluent and confluent cultures of a rat smooth muscle cell line. Exp Physiol 1992; 77(5):749–56.
Missiaen L, Oike M, Bootman MD, et al. Vasopressin responses in electrically coupled A7r5 cells. Pflugers Arch 1994; 428(3–4):283–7.
Bartlett IS, Crane GJ, Neild TO, Segal SS . Electrophysiological basis of arteriolar vasomotion in vivo. J Vasc Res 2000; 37(6):568–75.
Marks TN, Dubyak GR, Jones SW . Calcium currents in the A7r5 smooth muscle-derived cell line. Pflugers Arch 1990; 417(4):433–9.
Intaglietta M . Arteriolar vasomotion: implications for tissue ischemia. Blood Vessels 1991; 28(Suppl 1): 1–7.
Lee CH, Poburko D, Kuo KH, Seow CY, van Breemen C . Ca2+ oscillations, gradients, and homeostasis in vascular smooth muscle. Am J Physiol Heart Circ Physiol 2002; 282(5):H1571–83.
Neylon CB, Hoyland J, Mason WT, Irvine RF . Spatial dynamics of intracellular calcium in agonist-stimulated vascular smooth muscle cells. Am J Physiol 1990; 259(4 Pt 1):C675–86.
Wu SN, Yu HS, Seyama Y . Analytical studies of spontaneous and vasopressin-induced calcium oscillations in cultured vascular smooth muscle cells. J Biochem (Tokyo) 1996; 119(1):42–8.
Mahoney MG, Slakey LL, Hepler PK, Gross DJ . Independent modes of propagation of calcium waves in smooth muscle cells. J Cell Sci 1993; 104(Pt 4):1101–7.
Parys JB, Missiaen L, De Smedt H, Droogmans G, Casteels R . Bell-shaped activation of inositol-1,4,5-trisphosphate-induced Ca2+ release by thimerosal in permeabilized A7r5 smooth-muscle cells. Pflugers Arch 1993; 424(5–6):516–22.
Roullet JB, Le Quan Sang KH, Luft U, et al. Inhibition of Ca2+ uptake into A7r5 vascular smooth muscle cells by farnesol: lack of effect on membrane fluidity and Ca2+-ATPase activities. J Hypertens 1997; 15(12 Pt 2):1723–8.
Wu KD, Bungard D, Lytton J . Regulation of SERCA Ca2+ pump expression by cytoplasmic Ca2+ in vascular smooth muscle cells. Am J Physiol Cell Physiol 2001; 280(4):C843–51.
Byron KL, Taylor CW . Spontaneous Ca2+ spiking in a vascular smooth muscle cell line is independent of the release of intracellular Ca2+ stores. J Biol Chem 1993; 268(10):6945–52.
White C, McGeown JG . Inositol 1,4,5-trisphosphate receptors modulate Ca2+ sparks and Ca2+ store content in vas deferens myocytes. Am J Physiol Cell Physiol 2003; 285(1):C195–204.
Stehno-Bittel L, Sturek M . Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol (Lond) 1992; 451: 49–78.
Stehno-Bittel L, Laughlin MH, Sturek M . Exercise training depletes sarcoplasmic reticulum calcium in coronary smooth muscle. J Appl Physiol 1991; 71(5):1764–73.
Marsault R, Murgia M, Pozzan T, Rizzuto R . Domains of high Ca2+ beneath the plasma membrane of living A7r5 cells. Embo J 1997; 16(7):1575–81.
Zhang X, Wen Y, Xu X, Dong F, Ren J . Agonist-stimulated Ca2+ transport in mesenteric vascular smooth muscle cells of vitamin D(3)-induced calcium overload rats. Vascul Pharmacol 2003; 40(4):189–95.
Marie I, Beny JL . Calcium imaging of murine thoracic aorta endothelium by confocal microscopy reveals inhomogeneous distribution of endothelial cells responding to vasodilator agents. J Vasc Res 2002; 39(3):260–7.
Oishi H, Budel S, Schuster A, Stergiopulos N, Meister JJ, Beny JL . Cytosolic-free calcium in smooth-muscle and endothelial cells in an intact arterial wall from rat mesenteric artery in vitro. Cell Calcium 2001; 30(4):261–7.
Blatter LA, Wier WG . Agonist-induced [Ca2+]i waves and Ca2+-induced Ca2+ release in mammalian vascular smooth muscle cells. Am J Physiol 1992; 263(2 Pt 2):H576–86.
Cole L, Davies D, Hyde GJ, Ashford AE . ER-Tracker dye and BODIPY-brefeldin A differentiate the endoplasmic reticulum and golgi bodies from the tubular-vacuole system in living hyphae of Pisolithus tinctorius. J Microsc 2000; 197(Pt 3):239–49.
Bkaily G, Pothier P, D'Orleans-Juste P, et al. The use of confocal microscopy in the investigation of cell structure and function in the heart, vascular endothelium and smooth muscle cells. Mol Cell Biochem 1997; 172(1–2):171–94.
Goldman WF . Spatial and temporal resolution of serotonin-induced changes in intracellular calcium in a cultured arterial smooth muscle cell line. Blood Vessels 1991; 28(1–3):252–61.
Doyle VM, Ruegg UT . Vasopressin induced production of inositol trisphosphate and calcium efflux in a smooth muscle cell line. Biochem Biophys Res Commun 1985; 131(1):469–76.
Kaplan-Albuquerque N, Di Salvo J . Protein kinase C: modulation of vasopressin-induced calcium influx and release in A7r5 vascular smooth muscle cells. Arch Biochem Biophys 1998; 359(2):209–14.
Kimes BW, Brandt BL . Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res 1976; 98(2):349–66.
Van Renterghem C, Romey G, Lazdunski M . Vasopressin modulates the spontaneous electrical activity in aortic cells (line A7r5) by acting on three different types of ionic channels. Proc Natl Acad Sci U S A 1988; 85(23):9365–9.
Iino M, Kasai H, Yamazawa T . Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. Embo J 1994; 13(21):5026–31.
Haller H, Lindschau C, Quass P, Distler A, Luft FC . Nuclear calcium signaling is initiated by cytosolic calcium surges in vascular smooth muscle cells. Kidney Int 1994; 46(6):1653–62.
al-Mohanna FA, Caddy KW, Bolsover SR . The nucleus is insulated from large cytosolic calcium ion changes. Nature 1994; 367(6465):745–50.
Himpens B, De Smedt H, Casteels R . Subcellular Ca2+-gradients in A7r5 vascular smooth muscle. Cell Calcium 1994; 15(1):55–65.
Badminton MN, Kendall JM, Rembold CM, Campbell AK . Current evidence suggests independent regulation of nuclear calcium. Cell Calcium 1998; 23(2–3):79–86.
Stehno-Bittel L, Luckhoff A, Clapham DE . Calcium release from the nucleus by InsP3 receptor channels. Neuron 1995; 14(1):163–7.
Fujihara H, Fukuda S, Tanaka T, Kanazawa H, Fujiwara N, Shimoji K . Arginine vasopressin increases perinuclear [Ca2+] in single cultured vascular smooth muscle cells of rat aorta. J Vasc Res 1993; 30(4):231–8.
Stehno-Bittel L, Perez-Terzic C, Luckhoff A, Clapham DE . Nuclear ion channels and regulation of the nuclear pore. Soc Gen Physiol Ser 1996; 51: 195–207.
Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH . ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell 1995; 80(3):439–44.
Humbert JP, Matter N, Artault JC, Koppler P, Malviya AN . Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. Discrete distribution of inositol phosphate receptors to inner and outer nuclear membranes. J Biol Chem 1996; 271(1):478–85.
Bkaily G, Choufani S, Hassan G, et al. Presence of functional endothelin-1 receptors in nuclear membranes of human aortic vascular smooth muscle cells. J Cardiovasc Pharmacol 2000; 36(5 Suppl 1): S414–7.
Massaeli H, Hurtado C, Austria JA, Pierce GN . Increase in nuclear calcium in smooth muscle cells exposed to oxidized low density lipoprotein. Free Radic Res 2001; 34(1):9–16.
Byron KL . Vasopressin stimulates Ca2+ spiking activity in A7r5 vascular smooth muscle cells via activation of phospholipase A2. Circ Res 1996; 78(5):813–20.
Hill BJ, Wamhoff BR, Sturek M . Functional nucleotide receptor expression and sarcoplasmic reticulum morphology in dedifferentiated porcine coronary smooth muscle cells. J Vasc Res 2001; 38(5):432–43.
Fan J, Byron KL . Ca2+ signalling in rat vascular smooth muscle cells: a role for protein kinase C at physiological vasoconstrictor concentrations of vasopressin. J Physiol (Lond) 2000; 524(Pt 3):821–31.
Prakash YS, Pabelick CM, Kannan MS, Sieck GC . Spatial and temporal aspects of ACh-induced [Ca2+]i oscillations in porcine tracheal smooth muscle. Cell Calcium 2000; 27(3):153–62.
Berridge MJ, Lipp P, Bootman MD . The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000; 1(1):11–21.
Missiaen L, Robberecht W, van den Bosch L, et al. Abnormal intracellular ca2+ homeostasis and disease. Cell Calcium 2000; 28(1):1–21.
Acknowledgements
We greatly appreciate the work of Mrs. Eileen Roach for assistance with data collection. This work was supported by NIH RO1 GMS and US Department of Commerce SABIT grants to Lisa Stehno-Bittel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
FEDORYAK, O., SEARLS, Y., SMIRNOVA, I. et al. Spontaneous Ca2+ oscillations in subcellular compartments of vascular smooth muscle cells rely on different Ca2+ pools. Cell Res 14, 379–388 (2004). https://doi.org/10.1038/sj.cr.7290238
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/sj.cr.7290238
Keywords
This article is cited by
-
Nuclear pores enable sustained perinuclear calcium oscillations
BMC Systems Biology (2016)
-
Intracellular Ca2+ regulating proteins in vascular smooth muscle cells are altered with type 1 diabetes due to the direct effects of hyperglycemia
Cardiovascular Diabetology (2010)