Abstract
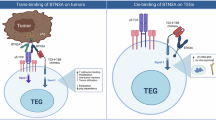
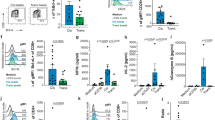
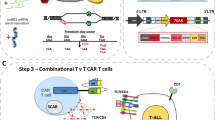
T cells require two distinct signals for optimal activation, an antigen-specific signal, provided by engagement of the T-cell receptor (TCR) and a second costimulatory signal mediated by engagement of CD28 with members of the B7 family. Although infiltrating T cells are present in many malignancies, they appear to be mostly anergic and do not attack the tumor, presumably because of the absence of activation and/or costimulatory signals. Here we describe a novel strategy for the in situ activation of tumor-specific T cells. We genetically modified T cells to secrete a bifunctional fusion protein, comprising the extracellular portion of B7-1 fused to an anticarcinoembryonic antigen (CEA) diabody. In coculture with CEA+ tumor cells autocrine and paracrine secretion of B7-αCEA provided a potent tumor-specific costimulatory signal to T cells in combination with a recombinant αCEA×αCD3 bispecific diabody. B7-αCEA was also found to strongly enhance survival and tumor-specific activation of T cells expressing an anti-CEA TCRζ–based chimeric immune receptor (CIR) both when expressed in cis by the T cells themselves as well as in trans, when added to the culture medium. In the absence of costimulatory signals provided by the tumor, our strategy allows T cells to “arm themselves” by the production of tumor-specific costimulatory proteins. Sustained in situ production of such molecules, like the B7-diabody fusion protein may create a favorable local environment for the activation and proliferation of tumor-reactive T cells and increase the tumoricidal activity of immunotherapeutic approaches targeting the TCR pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Townsend A, Bodmer H . Antigen recognition by class I–restricted T lymphocytes Annu Rev Immunol 1989 7: 601–624
Jenkins MK . The ups and downs of T cell costimulation Immunity 1994 1: 443–446
Tan P, Anasetti C, Hansen JA, Melrose J, Brunraud M, Bradshow J, Ledbetter J, Linsley PS . Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1 J Exp Med 1993 177: 165–173
Mueller DL, Jenkins MK, Schwartz RH . Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy Annu Rev Immunol 1989 7: 445–480
Linsley PS, Ledbetter JA . The role of the CD28 receptor during T cell responses to antigen Annu Rev Immunol 1993 11: 191–212
Allison JP . CD28-B7 interactions in T-cell activation Curr Opin Immunol 1994 6: 414–419
Chen L . Manipulation of T cell responses to tumors by targeting on costimulatory pathway Leukemia 1997 3: 567
Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS . Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4 Cell 1992 71: 1093–1102
Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellstrom I, Hellstrom KE . Tumor immunogenicity determines the effect of B7 costimulation on T cell–mediated tumor immunity J Exp Med 1994 179: 523–532
Álvarez-Vallina L, Hawkins RE . Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors Eur J Immunol 1996 26: 2304–2309
Álvarez-Vallina L . Targeted cell immunotherapy of human cancer: a promising approach that is extending its initial cell and molecular scope Tumor Targeting 2000 4: 225–234
Krause A, Guo HF, Latouche JB, Tan C, Cheung NK, Sadelain M . Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes J Exp Med 1998 188: 619–626
Eshhar Z, Waks T, Gross G, Schindler DG . Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors Proc Natl Acad Sci USA 1993 90: 720–724
Finney HM, Lawson AD, Bebbington CR, Weir AN . Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product J Immunol 1998 161: 2791–2797
Álvarez-Vallina L . Genetic approaches for antigen-selective therapy Curr Gene Ther 2001 1: 385–397
Gerstmayer B, Altenschmidt U, Hoffmann M, Wels W . Costimulation of T cell proliferation by a chimeric B7-2 antibody fusion protein specifically targeted to cells expressing the erbB2 proto-oncogene J Immunol 1997 158: 4584–4590
Rohrbach F, Gerstmayer B, Biburger M, Wells W . Construction and characterization of bispecific costimulatory molecules containing a minimized CD86 (B7-2) domain and single-chain antibody fragments for tumor targeting Clin Cancer Res 2000 6: 4314–4322
Holliger P, Manzke O, Span M et al. Carcinoembryonic antigen (CEA)-specific T-cell activation in colon carcinoma induced by anti-CD3×anti-CEA bispecific diabodies and B7×anti-CEA bispecific fusion proteins Cancer Res 1999 59: 2909–2916
Marshall KW, Marks JD . Engineering and characterization of a novel fusion protein incorporating B7.2 and an anti–ErbB-2 single-chain antibody fragment for the activation of Jurkat T cells J Immunother 2001 24: 27–36
Rosenberg SA, Spiess P, Lafreniere R . A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes Science 1986 233: 1318–1321
Bolhuis RL, Sturm E, Braakman E . T cell targeting in cancer therapy Cancer Immunol Immunother 1991 34: 1–8
Holliger P, Prospero T, Winter G . “Diabodies”: small bivalent and bispecific antibody fragments Proc Natl Acad Sci USA 1993 90: 6444–6448
Hochuli E, Bannwarth W, Döbeli H, Gentz R, Stüber D . Genetic approach to facilitate purification of recombinant proteins with a novel metal cHeLate adsorbent Biotechnology 1998 6: 1321
Álvarez-Vallina L, Agha-Mohammadi S, Hawkins RE, Rusell SJ . Pharmacological control of antigen responsiveness in genetically modified T lymphocytes J Immunol 1997 159: 5889–5895
Álvarez-Vallina L, González A, Gambón F, Kreisler M, Díaz-Espada F . Delimitation of the proliferative stages in the human thymus indicates that cell expansion occurs before the expression of CD3 (T cell receptor) J Immunol 1993 150: 8–16
Acknowledgements
This work was funded by the Comunidad Autónoma de Madrid Grant 08.1/0016/1998 (to L Álvarez-Vallina). B Blanco is supported by a Comunidad Autónoma de Madrid training Grant 01/0369/2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blanco, B., Holliger, P. & Álvarez-Vallina, L. Autocrine costimulation: Tumor-specific CD28-mediated costimulation of T cells by in situ production of a bifunctional B7–anti-CEA diabody fusion protein. Cancer Gene Ther 9, 275–281 (2002). https://doi.org/10.1038/sj.cgt.7700438
Received:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cgt.7700438
Keywords
This article is cited by
-
Balanced secretion of anti-CEA × anti-CD3 diabody chains using the 2A self-cleaving peptide maximizes diabody assembly and tumor-specific cytotoxicity
Gene Therapy (2017)
-
Inhibition of tumor growth in vivo by in situ secretion of bispecific anti-CEA × anti-CD3 diabodies from lentivirally transduced human lymphocytes
Cancer Gene Therapy (2007)