Abstract
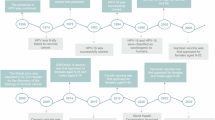
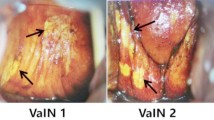
Human papillomavirus (HPV) is the etiologic agent for cervical cancer. In Mexico, a women dies every 2 h, and since 1990 the statistics have shown that the numbers of deaths are increasing. We conducted a phase II clinical trial to evaluate the potential use of the MVA E2 recombinant vaccinia virus in treating high-grade lesions (CIN 2 and CIN 3) associated with oncogenic papillomavirus. Fifty-four female patients with high degree lesions were treated either with an MVA E2 therapeutic vaccine or with conization. Thirty-four women received the therapeutic vaccine, at a total of 107 virus particles per dose injected directly into the uterus once every week over a 6-week period. Twenty control patients were treated with conization. By colposcopy, 19 patients out of 34 showed no lesion, in three patients the lesions were reduced by 85–90%, in eight other lesions had reduced by 60%, and in four more patients, they were reduced by 25%. Histological analysis showed total elimination of high-grade lesions in 20 out of 34 patients after treatment with MVA E2. Eleven patients had a 50% reduction in lesion size. In two other patients, the lesion was reduced to CIN 2 and in one more patient the lesion was reduced to low grade (CIN 1). All patients developed antibodies against the MVA E2 vaccine, and generated a specific cytotoxic response against papilloma-transformed cells. DNA viral load was significantly reduced in MVA E2-treated patients. Conization eliminated the lesions in 80% of the patients, but patients did not develop cytotoxic activity specific against cancer cells and did not eliminate the papillomavirus. In addition, three patients treated with conization had recurrence of lesions 1 year later. These results show that therapeutic vaccination with MVA E2 proved to be very effective in stimulating the immune system against papillomavirus, and in generating regression of high-grade lesion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Jansen K, Shaw A . Human papillomavirus vaccines and prevention of cervical cancer. Ann Rev Med 2004; 55: 319–331.
Parkin DM, Laara E, Muir CS . Estimates of the world-wide frequency of sixteen major cancers in 1980. Int J Cancer 1980; 41: 184–197.
Rosales C, Valadez G, Arrellin R, Merchant H, Rosales R . A recombinant vaccinia virus containing the papilloma E2 protein promotes tumor regression by stimulating macrophage antibody-dependent cytotoxicity. Cancer Immunol Immunother 2000; 49: 347–360.
Valadez V, Sutter G, Jose M, Garcia-Carranca A, Erfle V, Moreno M et al. Human tumor growth is inhibited by vaccinia virus carrying the E2 gene of bovine papillomavirus. Cancer 2000; 88: 1650.
Corona-Gutiérrez C, Tinoco A, Contreras M, Navarro T, Calzado P, Vargas L et al. A phase II study. Efficacy of the gene therapy of the MVA E2 recombinant virus in the treatment of precancerous lesions (NIC I and NIC II) associated with infection of oncogenic human papillomavirus. Hum Gene Ther 2002; 13: 1127–1140.
Corona-Gutiérrez C, Tinoco A, Navarro T, López-Contreras M, Risco-Cortes R, Calzado P et al. Therapeutic vaccination with MVA E2 can eliminate precancerous lesions (CIN 1, CIN 2 and CIN 3) associated with infection by oncogenic human papillomavirus. Hum Gene Ther 2004; 15: 421–431.
Binns MM, Smith GL . Recombinant Poxvirus. ACR Press: Boca Raton, Florida, 1993.
Sutter G, Wyatt LS, Foley PL, Bennick JR, Moss B . A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine 1994; 12: 53–59.
Roque-Reséndiz J, Rosales R, Herion P . MVA ROP 2 vaccinia virus recombinant as a vaccine candidate for toxoplasmosis. Parasitology 2004; 128: 397–405.
Hanke T, McMichael A, Mwau M, Wee EG, Ceberej I, Patel S et al. Development of a DNA-MVA/HIVA vaccine for Kenya. Vaccine 2002; 20: 1995–1998.
Wyatt L, Whitehead S, Venanzi K, Murphy B, Moss B . Priming and boosting immunity to respiratory syncytial virus by recombinant replication-defective vaccinia virus MVA. Vaccine 1999; 18: 392–397.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Hernández, E., González-Sánchez, J., Andrade-Manzano, A. et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther 13, 592–597 (2006). https://doi.org/10.1038/sj.cgt.7700937
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cgt.7700937
Keywords
This article is cited by
-
Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11–001
Gynecologic Oncology Research and Practice (2017)
-
Construction d’un vecteur d’expression chez Lactococcus lactis basée sur la production d’une protéine ancrée de Papillomavirus humain 16 E2/E7
Journal Africain du Cancer / African Journal of Cancer (2015)
-
Immunologic treatments for precancerous lesions and uterine cervical cancer
Journal of Experimental & Clinical Cancer Research (2014)
-
Cervical Cancer: Development of Targeted Therapies Beyond Molecular Pathogenesis
Current Obstetrics and Gynecology Reports (2014)
-
Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients
Nature Communications (2014)