Abstract
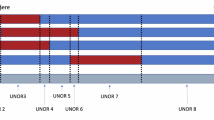
The Cohen syndrome is a rare autosomal recessively inherited disorder. Contrary to many case reports published elsewhere, the phenotype is uniform in Finland including nonprogressive mental and motor retardation, typical dysmorphic features, granulocytopenia and marked ophthalmological changes. By linkage analysis in five Finnish multiplex nuclear families, the COH1 locus for the Cohen syndrome was recently assigned to a 10-cM region between loci D8S270 and D8S521 on the long arm of chromosome 8. Here we present results of linkage disequilibrium and haplotype analysis in an extended panel of 16 Finnish COH1 families using new markers localized in the COH1 region. By inferring historical recombinations in conserved haplotypes the COH1 gene was assigned in the region of marker loci D8S1808, D8S1762 and D8S546. Calculations of genetic distances based on linkage disequilibrium suggest that the most likely localization of COH1 is in the immediate vicinity of marker locus D8S1762. Haplotype analysis suggests the occurrence of one main COH1 mutation and possibly one or two rare ones in Finland. This information will be useful in the positional cloning of the gene.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
McKusick VA: Mendelian Inheritance in Man. Catalog of Human Genes and Genetic Disorders, ed 11. Baltimore, Johns Hopkins University Press, 1994, vol 2, pp 1711–1712.
Cohen MM Jr, Hall BD, Smith DW, Graham CB, Lampert KJ: A new syndrome with hypotonia, obesity, mental deficiency, and facial, oral, ocular, and limb anomalies. J Pediatr 1973;83:280–284.
Carey JC, Hall BD: Confirmation of the Cohen syndrome. J Pediatr 1978;93:239–244.
Norio R, Raitta C, Lindahl E: Further delineation of the Cohen syndrome; report on chorioretinal dystrophy, leukopenia and consanguinity. Clin Genet 1984;25:1–14.
Resnick K, Zuckerman J, Cotlier E: Cohen syndrome with bull’s eye macular lesions. Ophthalmic Paediatr Genet 1986;7:1–18.
Warburg M, Pedersen SA, Hørlyk H: The Cohen syndrome: Retinal lesions and granulocytopenia. Ophthalmic Paediatr Genet 1990;11: 7–13.
Kondo I, Nagataki S, Miyagi N: The Cohen syndrome: Does mottled retina separate a Finnish and a Jewish type? Am J Med Genet 1990; 37:109–113.
Steinlein O, Tariverdian G, Boll HU, Vogel F: Tapetoretinal degeneration in brothers with apparent Cohen syndrome: Nosology with Mirhosseini-Holmes-Walton syndrome. Am J Med Genet 1991;41:196–200.
Tahvanainen E, Norio R, Karila E, Ranta S, Weissenbach J, Sistonen P, de la Chapelle A: Cohen syndrome gene assigned to the long arm of chromosome 8 by linkage analysis. Nat Genet 1994;7:201–204.
Hästbacka J, de la Chapelle A, Kaitila I, Sistonen P, Weaver A, Lander E: Linkage disequilibrium mapping in isolated populations: Diastrophic dysplasia in Finland. Nat Genet 1992; 2:204–211.
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning, a Laboratory Manual. Cold Spring Harbor, Cold Spring Harbor Laboratory Press, 1989.
Weissenbach J, Gyapay G, Dib C, Vignal A, Morisette J, Millasseau P, Vaysseix G, Lathrop M: A second-generation linkage map of the human genome. Nature 1992;359:794–801.
Gyapay G, Morissette J, Vignal A, Dib C, Fizames C, Millasseau P, Marc S, Bernardi G, Lathrop M, Weissenbach J: The 1993–94 Généthon human genetic linkage map. Nat Genet 1994;7:246–339.
Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J: A comprehensive genetic map of the human genome based on 5,264 micro-satellites. Nature 1996;380:152–154.
Weber JL, May PE: Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet 1989;44:388–396.
Bassam BJ, Caetano-Anolles G, Gresshoff PM: Fast and sensitive silver staining of DNA in Polyacrylamide gels. Anal Biochem 1991; 196: 80–83.
Lehesjoki AE, Koskiniemi M, Norio R, Tirrito S, Sistonen P, Lander E, de la Chapelle A: Localization of the EPM1 gene for progressive myoclonus epilepsy on chromosome 21: Linkage disequilibrium allows high resolution mapping. Hum Mol Genet 1993;2:1229–1234.
de la Chapelle A: Disease gene mapping in isolated human populations: The example of Finland. J Med Genet 1993;30:857–865.
Terwilliger JD: A powerful likelihood method for the analysis of linkage disequilibrium between trait loci and one or more polymorphic marker loci. Am J Hum Genet 1995;56:777–787.
Jorde LB: Linkage disequilibrium as a gene-mapping tool. Am J Hum Genet 1995;56:11–14.
Hästbacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, Coloma A, Lovett M, Buckler A, Kaitila I, Lander ES: The diastrophic dysplasia gene encodes a novel sulfate transporter: Positional cloning by fine-structure linkage disequilibrium mapping. Cell 1994;78:1073–1087.
Virtaneva K, Miao J, Träskelin AL, Stone N, Warrington JA, Weissenbach J, Myers KM, Cox DR, Sistonen P, de la Chapelle A, Lehesjoki AE: Progressive myoclonus epilepsy EPM1 locus maps to a 175-kb interval in distal 21q. Am J Hum Genet 1996;58:1247–1253.
Pennacchio La, Lehesjoki AE, Stone NE, Wilbur VL, Virtaneva K, Miao J, D’Amato E, Ramirez L, Faham M, Koskiniemi M, Warrington JA, Norio R, de la Chapelle A, Cox DR, Myers RM: Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1). Science 1996;271:1731–1734.
Mitchison HM, O’Rawe AM, Taschner PE, Sandkuijl LA, Santavuori P, de Vos N, Breuning MH, Mole SE, Gardiner RM, Järvelä IE: Batten disease gene, CLN3: Linkage disequilibrium mapping in the Finnish population, and analysis of European haplotypes. Am J Hum Genet 1995;56:654–662.
The International Batten Disease Consortium: Isolation of a novel gene underlying Batten disease, CLN3. Cell 1995;82:949–957.
Norio R: Diseases of Finland and Scandinavia; in Rotschild H (ed): Biocultural Aspects of Disease. New York, Academic Press, 1981, pp 359–415.
Virtaneva K, D’Amato E, Miao J, Koskiniemi M, Norio R, Avanzini G, Franceschetti S, Michelucci R, Tassinari CA, Omer S, Pennacchio LA, Myers RM, Dieguez-Lucena JL, Krane R, de la Chapelle A, Lehesjoki AE: Unstable minisatellite expansion causing recessively inherited myoclonus epilepsy, EPM1. Nat Genet 1997;15:393–396.
Ikonen E, Baumann M, Grön K, Syvänen AC, Enomaa N, Halila R, Aula P, Peltonen L: Aspartyglglucosaminuria: cDNA encoding human aspartylglucosaminidase and the missense mutation causing the disease. EMBO J 1991; 10:51–58.
Isoniemi A, Hietala M, Aula P, Jalanko A, Peltonen L: Identification of a novel mutation causing aspartylglucosaminuria reveals a mutation hotspot region in the aspartylglucosaminidase gene. Hum Mutat 1985;5:318–326.
Pierse A, Lyon M, Hampson IN, Cowling GJ, Gallagher JT: Molecular cloning of the major cell surface heparan sulfate proteoglycan from rat liver. J Biol Chem 1992;267:3894–3900.
Cole GJ, Burg M: Characterization of a heparan sulfate proteoglycan that copurifies with the neural cell adhesion molecule. Exp Cell Res 1989;182:44–60.
Gallagher JT, Lyon M, Steward WP: Structure and function of heparan sulphate proteoglycans. Biochem J 1986;236:313–325.
Stipp CS, Litwack ED, Lander AD: Cerebroglycan: An integral membrane heparan sulphate proteoglycan that is unique to the developing nervous system and expressed specifically during neuronal differentiation. J Cell Biol 1994; 124:149–160.
Schuler GD, Boguski MS, Stewart EA, Stein LD, Gyapay G, Rice K, White RE, Rodriguez-Tomé P, Aggarwal A, Bajorek E, Bentolila S, Birren BB, Butler A, Castle AB, Chiannilkulchai N, Chu A, Clee C, Cowles S, Day PJR, Dibling T, East C, Drouot N, Dunham I, Duprat S, Edwards C, Fan JB, Fang N, Fizames C, Garrett C, Green L, Hadley D, Harris M, Harrison P, Brady S, Hicks A, Holoway E, Hui L, Hussain S, Louis-Dit-Sully C, Ma J, MacGilvery A, Mader C, Maratukulam A, Matise TC, McKusick KB, Morissette J, Mungall A, Muselet D, Nusbaum HC, Page DC, Peck A, Perkins S, Piercy M, Qin F, Quackenbush J, Ranby S, Reif T, Rozen S, Sanders C, She X, Silva J, Slonim DK, Soderlund C, Sun WL, Tabar P, Thangarajah T, Vega-Czarny N, Vollrath D, Voyticky S, Wilmer T, Wu X, Adams MD, Auffray C, Walter NAR, Brandon R, Dehejia A, Goodfellow PN, Houlgatte R, Hudson JR Jr, Ide SE, Iorio KR, Lee WY, Seki N, Nagase T, Ishikawa K, Nomura N, Phillips C, Polymeropoulos MH, Sandusky M, Schmitt K, Berry R, Swanson K, Torres R, Venter JC, Sikela JM, Beckmann JS, Weissenbach J, Myers RM, Cox DR, James MR, Bentley D, Deloukas P, Lander ES, Hudson TJ: A gene map of the human genome. Science 1996;274:540–546.
Acknowledgements
We thank the COH1 families for cooperating in this study and Dr. Pertti Sistonen for help with statistical problems. This work was supported by the Academy of Finland, the Ulla Hjelt Fund of the Finnish Foundation for Pediatric Research, and the Finnish Cultural Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolehmainen, J., Norio, R., Kivitie-Kallio, S. et al. Refined Mapping of the Cohen Syndrome Gene by Linkage Disequilibrium. Eur J Hum Genet 5, 206–213 (1997). https://doi.org/10.1007/BF03405919
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1007/BF03405919