Abstract
Aim:
Peroxisome proliferator-activated receptor gamma (PPARγ) is a therapeutic target for obesity, cancer and diabetes mellitus. In order to develop potent lead compounds for obesity treatment, we screened a natural product library for novel PPARγ antagonists with inhibitory effects on adipocyte differentiation.
Methods:
Surface plasmon resonance (SPR) technology and cell-based transactivation assay were used to screen for PPARγ antagonists. To investigate the antagonistic mechanism of the active compound, we measured its effect on PPARγ/RXRα heterodimerization and PPARγ co-activator recruitment using yeast two-hybrid assay, Gal4/UAS cell-based assay and SPR based assay. The 3T3-L1 cell differentiation assay was used to evaluate the effect of the active compound on adipocyte differentiation.
Results:
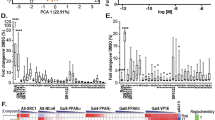
A new thiophene-acetylene type of natural product, 7-chloroarctinone-b (CAB), isolated from the roots of Rhaponticum uniflorum, was discovered as a novel PPARγ antagonist capable of inhibiting rosiglitazone-induced PPARγ transcriptional activity. SPR analysis suggested that CAB bound tightly to PPARγ and considerably antagonized the potent PPARγ agonist rosiglitazone-stimulated PPARγ-LBD/RXRα-LBD binding. Gal4/UAS and yeast two-hybrid assays were used to evaluate the antagonistic activity of CAB on rosiglitazone-induced recruitment of the coactivator for PPARγ. CAB could efficiently antagonize both hormone and rosiglitazone-induced adipocyte differentiation in cell culture.
Conclusion:
CAB shows antagonistic activity to PPARγ and can block the adipocyte differentiation, indicating it may be of potential use as a lead therapeutic compound for obesity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell 1999; 97: 161–3.
Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM . mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 1994; 8: 1224–34.
Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem 1997; 272: 18779–89.
Spiegelman BM . PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 1998; 47: 507–14.
Auwerx J . PPARgamma, the ultimate thrifty gene. Diabetologia 1999; 42: 1033–49.
Forman BM, Chen J, Evans RM . Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 1997; 94: 4312–7.
Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA 1997; 94: 4318–23.
Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol 1997; 11: 779–91.
Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM . Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998; 93: 229–40.
Ogden CL, Carroll MD, Flegal KM . High body mass index for age among US children and adolescents, 2003-2006. JAMA 2008; 299: 2401–5.
Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, et al. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Res 1994; 22: 5628–34.
Mukherjee R, Hoener PA, Jow L, Bilakovics J, Klausing K, Mais DE, et al. A selective peroxisome proliferator-activated receptor-gamma (PPARgamma) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol 2000; 14: 1425–33.
Yeh WC, Bierer BE, McKnight SL . Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3-L1 cells. Proc Natl Acad Sci USA 1995; 92: 11086–90.
Wright HM, Clish CB, Mikami T, Hauser S, Yanagi K, Hiramatsu R, et al. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J Biol Chem 2000; 275: 1873–7.
Camp HS, Chaudhry A, Leff T . A novel potent antagonist of peroxisome proliferator-activated receptor gamma blocks adipocyte differentiation but does not revert the phenotype of terminally differentiated adipocytes. Endocrinology 2001; 142: 3207–13.
Lee G, Elwood F, McNally J, Weiszmann J, Lindstrom M, Amaral K, et al. T0070907, a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J Biol Chem 2002; 277: 19649–57.
Rieusset J, Touri F, Michalik L, Escher P, Desvergne B, Niesor E, et al. A new selective peroxisome proliferator-activated receptor gamma antagonist with antiobesity and antidiabetic activity. Mol Endocrinol 2002; 16: 2628–44.
Liu HL, Guo YW . Three new thiophene acetylenes from Rhaponticum uniflorum (L.) DC. Helv Chim Acta 2008; 91: 130–5.
Ye F, Zhang ZS, Luo HB, Shen JH, Chen KX, Shen X, et al. The dipeptide H-Trp-Glu-OH shows highly antagonistic activity against PPARgamma: bioassay with molecular modeling simulation. Chembiochem 2006; 7: 74–82.
Zou G, Gao Z, Wang J, Zhang Y, Ding H, Huang J, et al. Deoxyelephantopin inhibits cancer cell proliferation and functions as a selective partial agonist against PPARgamma. Biochem Pharmacol 2008; 75: 1381–92.
Chen Q, Chen J, Sun T, Shen J, Shen X, Jiang H . A yeast two-hybrid technology-based system for the discovery of PPARgamma agonist and antagonist. Anal Biochem 2004; 335: 253–9.
Yeh WC, Cao Z, Classon M, McKnight SL . Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev 1995; 9: 168–81.
Hu E, Tontonoz P, Spiegelman BM . Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA 1995; 92: 9856–60.
Guardiola-Diaz HM, Rehnmark S, Usuda N, Albrektsen T, Feltkamp D, Gustafsson JA, et al. Rat peroxisome proliferator-activated receptors and brown adipose tissue function during cold acclimatization. J Biol Chem 1999; 274: 23368–77.
Hosono T, Mizuguchi H, Katayama K, Koizumi N, Kawabata K, Yamaguchi T, et al. RNA interference of PPARgamma using fiber-modified adenovirus vector efficiently suppresses preadipocyte-to-adipocyte differentiation in 3T3-L1 cells. Gene 2005; 348: 157–65.
Huang C, Zhang Y, Gong Z, Sheng X, Li Z, Zhang W, et al. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochem Biophys Res Commun 2006; 348: 571–8.
Liu QY, Quinet E, Nambi P . Adipocyte fatty acid-binding protein (aP2), a newly identified LXR target gene, is induced by LXR agonists in human THP-1 cells. Mol Cell Biochem 2007; 302: 203–13.
Nakamuta M, Enjoji M, Uchimura K, Ohta S, Sugimoto R, Kotoh K, et al. Bisphenol a diglycidyl ether (BADGE) suppresses tumor necrosis factor-alpha production as a PPARgamma agonist in the murine macrophage-like cell line, RAW 264.7. Cell Biol Int 2002; 26: 235–41.
Yamauchi T, Waki H, Kamon J, Murakami K, Motojima K, Komeda K, et al. Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J Clin Invest 2001; 108: 1001–13.
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (2006AA09Z447), National Natural Science Foundation of China (grants 30890044, 90713046, 20721003), Shanghai Pujiang Program PJ200700247.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Yt., Li, L., Chen, J. et al. 7-Chloroarctinone-b as a new selective PPARγ antagonist potently blocks adipocyte differentiation. Acta Pharmacol Sin 30, 1351–1358 (2009). https://doi.org/10.1038/aps.2009.113
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2009.113