Abstract
Aim:
Chickpea (Cicer arietinum L) is a traditional Uighur herb. In this study we investigated the estrogenic activities of the isoflavones extracted from chickpea sprouts (ICS) in ovariectomized rats.
Methods:
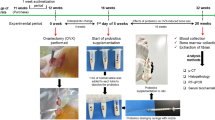
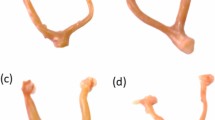
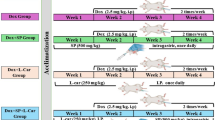
Ten-week-old virgin Sprague-Dawley female rats were ovariectomized (OVX). The rats were administered via intragastric gavage 3 different doses of ICS (20, 50, or 100 mg·kg−1·d−1) for 5 weeks. Their uterine weight and serum levels of 17β-estradiol (E2), follicle stimulating hormone (FSH) and luteinizing hormone (LH) were measured. The epithelial height, number of glands in the uterus, and number of osteoclasts in the femur were histologically quantified, and the expression of proliferating cell nuclear antigen (PCNA) was assessed immunohistochemically. Bone structural parameters, including bone mineral density (BMD), bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) were measured using Micro-CT scanning.
Results:
Treatments of OVX rats with ICS (50 or 100 mg·kg−1·d−1) produced significant estrogenic effects on the uteruses, including the increases in uterine weight, epithelial height and gland number, as well as in the expression of the cell proliferation marker PCNA. The treatments changed the secretory profile of ovarian hormones and pituitary gonadotropins: serum E2 level was significantly increased, while serum LH and FSH levels were decreased compared with the vehicle-treated OVX rats. Furthermore, the treatments significantly attenuated the bone loss, increased BMD, BV/TV and Tb.Th and decreased Tb.Sp and the number of osteoclasts. Treatment of OVX rats with the positive control drug E2 (0.25 mg·kg−1·d−1) produced similar, but more prominent effects.
Conclusion:
ICS exhibits moderate estrogenic activities as compared to E2 in ovariectomized rats, suggesting the potential use of ICS for the treatment of menopausal symptoms and osteoporosis caused by estrogen deficiency.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bryant HU, Dere WH . Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med 1998; 217: 45–52.
Manolagas SC . Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocrine Rev 2000; 21: 115–37.
Newton KM, Grady D . Soy isoflavones for prevention of menopausal bone loss and vasomotor symptoms. Arch Int Med 2011; 171: 1369–70.
Waldman TN . Menopause: When hormone replacement therapy is not an option. Part II. J Women Health 1998; 7: 673–83.
Barnabei VM, Grady D, Stovall DW, Cauley JA, Lin F, Stuenkel CA, et al. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstetric Gynecol 2002; 100: 1209–18.
Mei J, Yeung SSC, Kung AWC . High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab 2001; 86: 5217–21.
Steinberg FM, Murray MJ, Lewis RD, Cramer MA, Amato P, Young RL, et al. Clinical outcomes of a 2-yr soy isoflavone supplementation in menopausal women. Am J Clin Nutr 2011; 93: 356–67.
Dornstauder E, Jisa E, Unterrieder I, Krenn L, Kubelka W, Jungbauer A . Estrogenic activity of two standardized red clover extracts Menoflavon® intended for large scale use in hormone replacement therapy. J Steroid Biochem Mol Biol 2001; 78: 67–75.
Polan ML, Hochberg RB, Trant AS, Wuh HCK . Estrogen bioassay of ginseng extract and Arginmax®, a nutritional supplement for the enhancement of female sexual function. J Womens Health 2004; 13: 427–30.
Circosta C, De Pasquale R, Palumbo DR, Samperi S, Occhiuto F . Estrogenic activity of standardized extract of Angelica sinensis. Phytother Res 2006; 20: 665–9.
White PJ, Broadley MR . Biofortification of crops with seven mineral elements often lacking in human diets — iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist 2009; 182: 49–84.
Liu YM, Yikemu S . Wei Wu Er Yao Zhi. 1st ed. Urumqi (Xinjiang): People's Publishing House; 1986. p 469–71.
Zhao S, Zhang L, Gao P, Shao Z . Isolation and characterisation of the isoflavones from sprouted chickpea seeds. Food Chem 2009; 114: 869–73.
Cheng Z, Ayiguli A, Ma Q, Gao Y, Aisa HA . Determination of isoflavones in extracts of cicer afiefinum L and bean sprout by ultrabiolet spectrophotometry. Lishizhen Med Mater Med Res 2008; 19: 2612–3.
Lv Q, Yang Y, Zhao Y, Gu D, He D, Yili A, et al. Comparative study on separation and purification of isoflavones from the seeds and sprouts of chickpea by high-speed countercurrent chromatography. J Liq Chromatogr R T 2009; 32: 2879–92.
Zava DT, Dollbaum CM, Blen M . Estrogen and progestin bioactivity of foods, herbs, and spices. Proc Soc Exp Biol Med 1998; 217: 369–78.
Wei H, Yili A, Ma Q, Mai D, Wang Z, Ma H . Establishment and application of co-transfection screening method for phytoestrogen active constituents. Zhongguo Zhong Yao Za Zhi 2011; 36: 2530–4.
Diel P, Schulz T, Smolnikar K, Strunck E, Vollmer G, Michna H . Ability of xeno- and phytoestrogens to modulate expression of estrogen-sensitive genes in rat uterus: estrogenicity profiles and uterotropic activity. J Steroid Biochem Mol Biol 2000; 73: 1–10.
Diel P, Smolnikar K, Schulz T, Laudenbach-Leschowski U, Michna H, Vollmer G . Phytoestrogens and carcinogenesis — differential effects of genistein in experimental models of normal and malignant rat endometrium. Hum Reprod 2001; 16: 997–1006.
Möller FJ, Diel P, Zierau O, Hertrampf T, Maass J, Vollmer G . Long-term dietary isoflavone exposure enhances estrogen sensitivity of rat uterine responsiveness mediated through estrogen receptor α. Toxicol Lett 2010; 196: 142–53.
Li W, Liu YH . Effects of phytoestrogen genistein on genioglossus function and oestrogen receptors expression in ovariectomized rats. Arch Oral Biol 2009; 54: 1029–34.
Malaivijitnond S, Kiatthaipipat P, Cherdshewasart W, Watanabe G, Taya K . Different effects of Pueraria mirifica, a herb containing phytoestrogens, on LH and FSH secretion in gonadectomized female and male rats. J Pharmacol Sci 2004; 96: 428–35.
Arai K, Komura H, Akikusa T, Iio K, Kishi H, Watanabe G, et al. Contributions of endogenous inhibin and estradiol to the regulation of follicle-stimulating hormone and luteinizing hormone secretion in the pregnant rat. Biol Reproduction 1997; 56: 1482–89.
Ishida H, Uesugi T, Toda T, Tsuji K . Effects of soy isoflavones, daidzein, genistein and glycitein, on bone loss and lipid metabolic pathway in ovariectomized rats. J Nutr 2000; 130: 685S–6S.
Clifton-Bligh PB, Baber RJ, Fulcher GR, Nery ML, Moreton T . The effect of isoflavones extracted from red clover (Rimostil) on lipid and bone metabolism. Menopause 2001; 8: 259–65.
Wang XX, Wu J, Chiba H, Umegaki K, Yamada K, Ishimi Y . Puerariae radix prevents bone loss in ovariectomized mice. J Bone Mine Metab 2003; 21: 268–75.
Acknowledgements
We thank Ying-qin LI, Hai-qing ZHAO, Yan-hong LI, Chun-fang LU, and Mei MA at the Animal Experimental Center, First Teaching Hospital Xinjiang Medical University for their assistance with rat surgery. We thank Prof Yiu-fai CHEN of the University of Alabama for language revision of the manuscript. This work was supported in part by grants from the China National Funds for Distinguished Young Scientists (Grant No 30925045), National Natural Science Foundation of China (Grant No 81102890), CAS/SAFEA International Partnership Program for Creative Research Teams (Grant No KGCX2-YW-503) and Natural Science Foundation of Xinjiang Province, China (Grant No 2010211A58).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, Hr., Wang, J., Qi, Hx. et al. Assessment of the estrogenic activities of chickpea (Cicer arietinum L) sprout isoflavone extract in ovariectomized rats. Acta Pharmacol Sin 34, 380–386 (2013). https://doi.org/10.1038/aps.2012.160
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.160
Keywords
This article is cited by
-
The effect of Momordica charantia intake on the estrogen receptors ESRα/ESRβ gene levels and apoptosis on uterine tissue in ovariectomy rats
Molecular Biology Reports (2015)
-
Discovery of novel aromatase inhibitors using a homogeneous time-resolved fluorescence assay
Acta Pharmacologica Sinica (2014)