Abstract
Aim:
To construct a lentivirus-based inhibitor with specific secondary structure that could exert long-term suppression on microRNA-338-3p (miR-338-3p), thus elucidating its molecular function in colorectal carcinoma cells.
Methods:
The miR-338-3p inhibitor sequence was synthesized and inserted into pLV-THM plasmid. HEK-293T cells were co-transfected with the lentiviral vectors pLV-THM-miR-338-3p-inhibitor, psPAX2, and pMD2.G. The supernatant containing the lentivirus particles was harvested to determine the viral titer, and then used to infect colorectal carcinoma-derived SW-620 cells. eGFP(+) cells were sorted using flow cytometry. The expression of miR-338-3p in SW-620 cells was determined with real-time RT-PCR, and the expression of the smoothened (SMO) protein was detected using Western blot analysis. The migration ability of the transfected SW-620 cells was assessed with transwell assay.
Results:
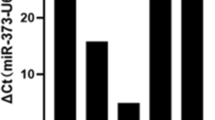
Restriction endonuclease analysis and DNA sequencing demonstrated that the lentiviral vector pLV-THM-miR-338-3p-inhibitor was successfully constructed. The expression of miR-338-3p in SW-620 cells was significantly decreased by infection with the lentivirus pLV-THM-miR-338-3p-inhibitor. Moreover, the down-regulated expression of miR-338-3p caused up-regulated expression of the SMO protein in SW-620 cells, which showed significantly enhanced migration in transwell assay.
Conclusion:
The construction of the lentiviral vector pLV-THM-miR-338-3p-inhibitor with specific secondary structure provides a basis for further studies the molecular function of miR-338-3p in colorectal carcinoma. miR-338-3p may suppress SMO gene expression and thereby inhibit colorectal carcinoma migration.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bartels CL, Tsongalis GJ . MicroRNAs: novel biomarkers for human cancer. Clin Chem 2009; 55: 623–31.
Mirnezami AH, Pickard K, Zhang L, Primrose JN, Packham G . MicroRNAs: key players in carcinogenesis and novel therapeutic targets. Eur J Surg Oncol 2009; 35: 339–47.
Torres AG, Fabani MM, Vigorito E, Williams D, Al-Obaidi N, Wojciechowski F, et al. Chemical structure requirements and cellular targeting of microRNA-122 by peptide nucleic acids anti-miRs. Nucleic Acids Res 2012; 40: 2152–67.
Ebert MS, Sharp PA . MicroRNA sponges: progress and possibilities. RNA 2010; 16: 2043–50.
Ebert MS, Neilson JR, Sharp PA . MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 2007; 4: 721–6.
Ebert MS, Sharp PA . Emerging roles for natural microRNA sponges. Curr Biol 2010; 20: 858–61.
Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ . A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci U S A 2005; 102: 13212–7.
Reichel M, Li J, Millar AA . Silencing the silencer: strategies to inhibit microRNA activity. Biotechnol Lett 2011; 33: 1285–92.
Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, Kaplan BB . MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J Neurosci 2008; 28: 12581–90.
Park F . Lentiviral vectors: are they the future of animal transgenesis? Physiol Genomics 2007; 31: 159–73.
Vermeulen A, Robertson B, Dalby AB, Marshall WS, Karpilow J, Leake D, et al. Double-stranded regions are essential design components of potent inhibitors of RISC function. RNA 2007; 13: 723–30.
Haraguchi T, Nakano H, Tagawa T, Ohki T, Ueno Y, Yoshida T, et al. A potent 2′-O-methylated RNA-based microRNA inhibitor with unique secondary structures. Nucleic Acids Res 2012; 40: e58.
Tokusumi Y, Ma Y, Song X, Jacobson RH, Takada S . The new core promoter element XCPE1 (X Core Promoter Element 1) directs activator-, mediator-, and TATA-binding protein-dependent but TFIID-independent RNA polymerase II transcription from TATA-less promoters. Mol Cell Biol 2007; 27: 1844–58.
Sun K, Zeng JJ, Wang W, Wu CT, Lei ST, Li GX . MicroRNA-221 inhibits CDKN1C/p57 expression in human colorectal carcinoma. Acta Pharmacol Sin 2011; 32: 375–84.
Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A . Identification of mammalian microRNA host genes and transcription units. Genome Res 2004; 14: 1902–10.
Tsuchiya S, Oku M, Imanaka Y, Kunimoto R, Okuno Y, Terasawa K, et al. MicroRNA-338-3p and microRNA-451 contribute to the formation of basolateral polarity in epithelial cells. Nucleic Acids Res 2009; 37: 3821–7.
Huang XH, Wang Q, Chen JS, Fu XH, Chen XL, Chen LZ, et al. Bead-based microarray analysis of microRNA expression in hepatocellular carcinoma: miR-338 is down-regulated. Hepatology Research 2009; 39: 786–94.
Hu B, Tai A, Wang P . Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol Rev 2011; 239: 45–61.
Yue J . miRNA and vascular cell movement. Adv Drug Deliv Rev 2011; 63: 616–22.
Haraguchi T, Ozaki Y, Iba H . Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Res 2009; 37: e43.
Scherr M, Venturini L, Battmer K, Schaller-Schoenitz M, Schaefer D, Dallmann I, et al. Lentivirus-mediated antagomir expression for specific inhibition of miRNA function. Nucleic Acids Res 2007; 35: e149.
Wang K, Pan L, Che X, Cui D, Li C . Sonic Hedgehog/GLI1 signaling pathway inhibition restricts cell migration and invasion in human gliomas. Neurol Res 2010; 32: 975–80.
Huang XH, Chen JS, Wang Q, Chen XL, Wen L, Chen LZ, et al. miR-338-3p suppresses invasion of liver cancer cell by targeting smoothened. J Pathol 2011; 225: 463–72.
Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A 2004; 101: 10380–5.
Acknowledgements
This research is supported by a grant from the National Natural Science Foundation of China (No 81101896).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, K., Guo, C., Deng, Hj. et al. Construction of lentivirus-based inhibitor of hsa-microRNA-338-3p with specific secondary structure. Acta Pharmacol Sin 34, 167–175 (2013). https://doi.org/10.1038/aps.2012.172
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.172