Abstract
Aim:
Lactoferrin (LF), an 80-kDa iron-binding glycoprotein, is a pleiotropic factor found in colostrum, milk, saliva and epithelial cells of the exocrine glands. The aim of this study was to evaluate the effects of LF on the bones in ovariectomized (Ovx) rats and to identify the pathways that mediate the anabolic action of LF on the bones.
Methods:
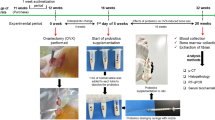
Female Sprague-Dawley rats (6-month-old) underwent ovariectomy, and were treated with different doses of LF (10, 100, 1000, and 2000 mg·kg−1·d−1, po) or with 7β-estradiol (0.1 mg·kg−1, im, each week) as the positive control. By the end of 6 month-treatments, the bone mass and microstructure in the rats were scanned by micro-computed tomography (micro-CT), and the bone metabolism was evaluated with specific markers, and the mRNA levels of osteoprotegerin (OPG) and the receptor-activator of nuclear factor κB ligand (RANKL) in femur were measured using qRT-PCR.
Results:
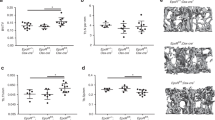
LF treatment dose-dependently elevated the bone volume (BV/TV), trabecular thickness (TbTh) and trabecular number (TbN), and reduced the trabecular separation (TbSp) in Ovx rats. Furthermore, higher doses of LF (1000 and 2000 mg·kg−1·d−1) significantly increased the bone mineral density (BMD) compared with the untreated Ovx rats. The higher doses of LF also significantly increased the serum levels of OC and BALP, and decreased the serum levels of β-CTx and NTX. LF treatment significantly increased the OPG mRNA levels, and suppressed the RANKL mRNA levels, and the RANKL/OPG mRNA ratio in Ovx rats.
Conclusion:
Oral administration of LF preserves the bone mass and improves the bone microarchitecture. LF enhances bone formation, reduces bone resorption, and decreases bone mass loss, possibly through the regulation of OPG/RANKL/RANK pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Garnero P, Delmas PD, Bilezikian JP . Evaluation of risk for osteoporosis fractures. Principles of Bone Biology 2002; 1291–301.
Naot D, Grey A, Reid IR, Cornish J . Lactoferrin — a novel bone growth factor. Clin Med Res 2005; 5: 93–101.
Omi N, Ezawa I . The effect of ovariectomy on bone metabolism in rats. Bone 1995; 17: 163S–168S.
Metz-Boutigue MH, Jollès J, Mazurier J, Schoentgen F, Legrand D, Spik G, et al. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem 1984; 145: 659–76.
Lonnerdal B, Iyer S . Lactoferrin: molecular structure and biological function. Annu Rev Nutr 1995; 15: 93–110.
Ward PP, Paz E, Conneely OM . Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci 2005; 62: 2540–8.
Tomita M, Wakabayashi H, Shin K, Yamauchi K, Yaeshima T, Iwatsuki K . Twenty-five years of research on bovine lactoferrin applications. Biochimie 2009; 91: 52–7.
Cornish J, Callon KE, Naot D, Palmano KP, Banovic T, Bava U, et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 2004; 145: 4366–74.
Grey A, Banovic T, Zhu Q, Watson M, Callon K, Palmano K, et al. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol 2004; 18: 2268–78.
Grey A, Zhu Q, Watson M, Callon K, Cornish J . Lactoferrin potently inhibits osteoblast apoptosis, via an LRP1-independent pathway. Mol Cell Endocrinol 2006; 251: 96–102.
Lorget F, Clough J, Oliveira M, Daury MC, Sabokbar A, Offord E . Lactoferrin reduces in vitro osteoclast differentiation and resorbing activity. Biochem Biophys Res Commun 2002; 296: 261–6.
Takayama Y, Mizumachi K . Effect of lactoferrin-embedded collagen membrane on osteogenic differentiation of human osteoblast-like cells. J Biosci Bioeng 2009; 107: 191–5.
Naot D, Grey A, Reid IR, Cornish J . Lactoferrin: a novel bone growth factor. Clin Med Res 2005; 3: 93–101.
Guo HY, Jiang L, Ibrahim SA, Zhang L, Zhang H, Zhang M, et al. Orally administered lactoferrin preserves bone mass and microarchitecture in ovariectomized rats. J Nutr 2009; 139: 958–64.
Bharadwaj S, Naidu AG, Betageri GV, Prasadarao NV, Naidu AS . Milk ribonuclease-enriched-lactoferrin induces positive effects on bone turnovermarkers in postmenopausal women. Osteoporos Int 2009; 20: 1603–11.
Boyle WJ, Simonet WS, Lacey DL . Osteoclast differentiation and activation. Nature 2003; 423: 337–42.
Mitchner NA, Harris ST . Current and emerging therapies for osteoporosis. J Fam Pract 2009; 58: S45–9.
Amizuka N, Shimomura J, Li M, Seki Y, Oda K, Henderson JE, et al. Defective bone remodelling in osteoprotegerin-deficien mice. J Electron Microsc (Tokyo) 2003; 52: 503–13.
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev 1999; 13: 2412–24.
Manolagos SC, Jilka RL . Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 1995; 332: 305–11.
Blais A, Malet A, Mikogami T, Martin-Rouas C, Tomé D . Oral bovine lactoferrin improves bone status of ovariectomized mice. Am J Physiol Endocrinol Metab 2009; 296: E1281–8.
Chomczynski P, Sacchi N . Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162: 156–9.
Melton LJ 3rd, Khosla S, Atkinson EJ, O'Fallon WM, Riggs BL . Relationship of bone turnover to bone density and fractures. J Bone Miner Res 1997; 12: 1083–91.
Schroeder TM, Jensen ED, Westendorf JJ . Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today 2005; 75: 213–25.
Marie PJ . Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys 2008; 473: 98–105.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998; 93: 165–76.
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999; 397: 315–23.
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997; 89: 309–19.
Rauner M, Sipos W, Pietschmann P . Osteoimmunology. Int Arch Allergy Immunol 2007; 143: 31–48.
Sandberg WJ, Yndestad A, Øie E, Smith C, Ueland T, Ovchinnikova O, et al. Enhanced t-cell expression of rank ligand in acute coronary syndrome: possible role in plaque destabilization. Arterioscler Thromb Vasc Biol 2006; 26: 857–63.
Acknowledgements
This study was supported by Fujian Science & Technology Major Special Project (No 2010Y0015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, Jm., Xue, Y. & Lin, Qm. Bovine lactoferrin improves bone mass and microstructure in ovariectomized rats via OPG/RANKL/RANK pathway. Acta Pharmacol Sin 33, 1277–1284 (2012). https://doi.org/10.1038/aps.2012.83
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.83
Keywords
This article is cited by
-
Association between Dairy Product intake and Risk of Osteoporotic Fractures in Postmenopausal Japanese Women: Secondary Analysis of 15-Year Follow-Up data from the Japanese Population-Based Osteoporosis (JPOS) Cohort Study
The Journal of nutrition, health and aging (2023)
-
Role of Iron Accumulation in Osteoporosis and the Underlying Mechanisms
Current Medical Science (2023)
-
Lactoferrin promotes the autophagy activity during osteoblast formation via BCL2-Beclin1 signaling
Molecular Biology Reports (2022)
-
RNA sequencing analysis reveals increased expression of interferon signaling genes and dysregulation of bone metabolism affecting pathways in the whole blood of patients with osteogenesis imperfecta
BMC Medical Genomics (2020)
-
Oral administration of bovine lactoferrin accelerates the healing of fracture in ovariectomized rats
Journal of Bone and Mineral Metabolism (2020)