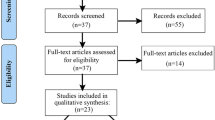
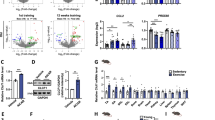
Abstract
Aim:
To study the roles of autophagy in muscle establishment during long-term exercise in mice.
Methods:
Female ICR mice were submitted to exercise training with a wheel running regimen: 6 m/min, 15 min/time, 3 times/d (on 8:00, 14:00, and 20:00), 5 d/week for 2 months. The mice were treated with the autophagy activator trehalose (1% aqueous solution as a daily drinking water) or the autophagy inhibitor chloroquine (10 mg/kg, ip, 5 times a week) before the training. Western blotting analysis, TUNEL staining, H&E staining and transmission electron microscope were used to evaluate the activity of autophagy and the structure of the muscle fibers.
Results:
The exercise training significantly stimulated the formation of autophagosomes, increased the LC3-II, cathepsin L and Bcl-2 levels, lowered the P62 level and increased the antioxidant capacity in the muscles. Meanwhile, the exercise training significantly improved the morphology of mitochondria, reduced the release of cytochrome c from mitochondria to cytoplasm, and slightly decreased the apoptosis rate in the muscles. Administration of trehalose increased the level of autophagy and protected the muscle fibers from apoptosis. Administration of chloroquine blocked autophagy flux and exerted detrimental effects on the muscles, which were ameliorated by the exercise training.
Conclusion:
Long-term regular exercise activates autophagy process associated with muscle establishment, and ameliorates the detrimental effects of chloroquine on skeletal muscles via restoring autophagy flux.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Warburton DE, Nicol CW, Bredin SS . Health benefits of physical activity: the evidence. CMAJ 2006; 174: 801–9.
Mizushima N, Levine B, Cuervo AM, Klionsky DJ . Autophagy fights disease through cellular self-digestion. Nature 2008; 451: 1069–75.
Sandri M . Autophagy in skeletal muscle. FEBS Lett 2010; 584: 1411–6.
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, et al. Autophagy is required to maintain muscle mass. Cell Metabolism 2009; 10: 507–15.
Fukuda T, Ahearn M, Roberts A, Mattaliano RJ, Zaal K, Ralston E, et al. Autophagy and mistargeting of therapeutic enzyme in skeletal muscle in Pompe disease. Mol Ther 2006; 14: 831–9.
Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, et al. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol 2010; 20: 143–8.
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, et al. Autophagy is required to maintain muscle mass. Cell Metab 2009; 10: 507–15.
Sung YH, Kim SC, Hong HP, Park CY, Shin MS, Kim CJ, et al. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson's disease mice. Life Sci 2012; 91: 1309–16.
Schenkman M, Hall DA, Baron AE, Schwartz RS, Mettler P, Kohrt WM . Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther 2012; 92: 1395–410.
Ogura Y, Iemitsu M, Naito H, Kakigi R, Kakehashi C, Maeda S, et al. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem Biophys Res Commun 2011; 414: 756–60.
Kim YA, Kim YS, Song W . Autophagic response to a single bout of moderate exercise in murine skeletal muscle. J Physiol Biochem 2011; 68: 229–35.
Jamart C, Benoit N, Raymackers JM, Kim HJ, Kim CK, Francaux M . Autophagy-related and autophagy-regulatory genes are induced in human muscle after ultraendurance exercise. Eur J Appl Physiol 2012; 112: 3173–7.
Wohlgemuth SE, Lees HA, Marzetti E, Manini TM, Aranda JM, Daniels MJ, et al. An exploratory analysis of the effects of a weight loss plus exercise program on cellular quality control mechanisms in older overweight women. Rejuvenation Res 2011; 14: 315–24.
He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 2012; 481: 511–5.
Irrcher I, Adhihetty PJ, Joseph AM, Ljubicic V, Hood DA . Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med 2003; 33: 783–93.
Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 2009; 1: 425–37.
Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab 2008; 8: 425–36.
Pietri-Rouxel F, Gentil C, Vassilopoulos S, Baas D, Mouisel E, Ferry A, et al. DHPR alpha1S subunit controls skeletal muscle mass and morphogenesis. EMBO J 2010; 29: 643–54.
Tolkovsky AM . Autophagy thwarts muscle disease. Nat Med 2010; 16: 1188–90.
Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC . Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 2007; 282: 5641–52.
Yang YP, Hu LF, Zheng HF, Mao CJ, Hu WD, Xiong KP, et al. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol Sin 2013; 34: 625–35.
Suzuki T, Nakagawa M, Yoshikawa A, Sasagawa N, Yoshimori T, Ohsumi Y, et al. The first molecular evidence that autophagy relates rimmed vacuole formation in chloroquine myopathy. J Biochem 2002; 131: 647–51.
Ramos L, Wetzels AM . Low rates of DNA fragmentation in selected motile human spermatozoa assessed by the TUNEL assay. Hum Reprod 2001; 16: 1703–7.
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441: 880–4.
Raben N, Hill V, Shea L, Takikita S, Baum R, Mizushima N, et al. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum Mol Genet 2008; 17: 3897–908.
Criscuolo F, Gonzalez-Barroso Mdel M, Le Maho Y, Ricquier D, Bouillaud F . Avian uncoupling protein expressed in yeast mitochondria prevents endogenous free radical damage. Proc Biol Sci 2005; 272: 803–10.
Alper G, Girgin FK, Ozgonul M, Mentes G, Ersoz B . MAO inhibitors and oxidant stress in aging brain tissue. Eur Neuropsychopharmacol 1999; 9: 247–52.
Elmore S . Apoptosis: a review of programmed cell death. Toxicol Pathol 2007; 35: 495–516.
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G . Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 2006; 13: 1423–33.
Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C . Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem 2005; 280: 35742–50.
Girard I, McAleer MW, Rhodes JS, Garland T Jr. . Selection for high voluntary wheel-running increases speed and intermittency in house mice (Mus domesticus). J Exp Biol 2001; 204: 4311–20.
Colaco C, Kampinga J, Roser B . Amorphous stability and trehalose. Science 1995; 268: 788.
Tanaka M, Machida Y, Niu S, Ikeda T, Jana NR, Doi H, et al. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 2004; 10: 148–54.
Salminen A, Vihko V . Autophagic response to strenuous exercise in mouse skeletal muscle fibers. Virchows Arch B Cell Pathol Incl Mol Pathol 1984; 45: 97–106.
Acknowledgements
We are grateful to Dr Fiona M MENZIES for her careful revision and critical comments on this paper. This work were supported by grants from the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADA) and Jiangsu Key Laboratory of Translational Research and Therapy for Neuro-Psycho-Diseases and College of Pharmaceutical Sciences, Soochow University, China (BM2013003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, D., Chen, K., Lu, X. et al. Exercise ameliorates the detrimental effect of chloroquine on skeletal muscles in mice via restoring autophagy flux. Acta Pharmacol Sin 35, 135–142 (2014). https://doi.org/10.1038/aps.2013.144
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.144
Keywords
This article is cited by
-
Long-term running exercise alleviates cognitive dysfunction in APP/PSEN1 transgenic mice via enhancing brain lysosomal function
Acta Pharmacologica Sinica (2022)
-
Long-term resistance exercise-induced muscular hypertrophy is associated with autophagy modulation in rats
The Journal of Physiological Sciences (2018)
-
The beneficial role of proteolysis in skeletal muscle growth and stress adaptation
Skeletal Muscle (2016)
-
Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington's disease
Acta Pharmacologica Sinica (2015)