Abstract
Aim:
6-Shogaol [1-(4-hydroxy-methoxyphenyl)-4-decen-one], a pungent compound isolated from ginger, has shown various neurobiological and anti-inflammatory effects. The aim of this study was to examine the effects of 6-shogaol on neuroinflammatory-induced damage of dopaminergic (DA) neurons in Parkinson's disease (PD) models.
Methods:
Cultured rat mesencephalic cells were treated with 6-shogaol (0.001 and 0.01 μmol/L) for 1 h, then with MPP+(10 μmol/L) for another 23 h. The levels of TNF-α and NO in medium were analyzed spectrophotometrically. C57/BL mice were administered 6-shogaol (10 mg·kg−1·d−1, po) for 3 d, and then MPTP (30 mg/kg, ip) for 5 d. Seven days after the last MPTP injection, behavioral testings were performed. The levels of tyrosine hydroxylase (TH) and macrophage antigen (MAC)-1 were determined with immunohistochemistry. The expression of iNOS and COX-2 was measured using RT PCR.
Results:
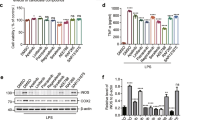
In MPP+-treated rat mesencephalic cultures, 6-shogaol significantly increased the number of TH-IR neurons and suppressed TNF-α and NO levels. In C57/BL mice, treatment with 6-shogaol reversed MPTP-induced changes in motor coordination and bradykinesia. Furthermore, 6-shogaol reversed MPTP-induced reductions in TH-positive cell number in the substantia nigra pars compacta (SNpc) and TH-IR fiber intensity in stratum (ST). Moreover, 6-shogaol significantly inhibited the MPTP-induced microglial activation and increases in the levels of TNF-α, NO, iNOS, and COX-2 in both SNpc and ST.
Conclusion:
6-Shogaol exerts neuroprotective effects on DA neurons in in vitro and in vivo PD models.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ransohoff RM, Perry VH . Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 2009; 27: 119–45.
Perry VH, Nicoll JAR, Holmes C . Microglia in neurodegenerative disease. Nat Rev Neurol 2010; 6: 193–201.
Przedborski S . Neuroinflammation and Parkinson's disease. Handb Clin Neurol 2007; 83: 535–51.
Schwartz M, Shechter R . Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol 2010; 6: 405–10.
Zick SM, Djuric Z, Ruffin MT, Litzinger AJ, Normolle DP, Alrawi S, et al. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 2008; 17: 1930–6.
Chen CC, Kuo MC, Ho CT . High performance liquid chromatographic determination of pungent gingerol compounds of ginger (Zingiber officinale Roscoe). J Food Sci 1986; 51: 1364–65.
Asami A, Shimada T, Mizuhara Y, Asano T, Takeda S, Aburada T, et al. Pharmacokinetics of 6-shogaol, a pungent ingredient of Zingiber officinale Roscoe (Part I). J Nat Med 2010; 64: 281–7.
Kyung KS, Gon JH, Geun KY, Sup JJ, Suk WJ, Ho KJ . 6-Shogaol, a natural product, reduces cell death and restores motor function in rat spinal cord injury. Eur J Neurosci 2006; 24: 1042–52.
Pertz HH, Lehmann J, Roth-Ehrang R, Elz S . Effects of ginger constituents on the gastrointestinal tract: role of cholinergic M3 and serotonergic 5-HT3 and 5-HT4 receptors. Planta Med 2011; 77: 973–8.
Shim S, Kim S, Kwon YB, Kwon J . Protection by 6-shogaol against lipopolysaccharide-induced toxicity in murine astrocytes is related to production of brain-derived neurotrophic factor. Food Chem Toxicol 2011; 50: 597–602.
Shim S, Kwon J . Effects of 6-shogaol on cholinergic signaling in HT22 cells following neuronal damage induced by hydrogen peroxide. Food Chem Toxicol 2012; 50: 1454–9.
Ha SK, Moon E, Ju MS, Kim DH, Ryu JH, Oh MS, et al. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012; 63: 211–23.
Tieu K . A guide to neurotoxic animal models of Parkinson's disease. Cold Spring Harbor Perspect Med 2011; 1: a009316.
Przedborski S, Vila M . MPTP: a review of its mechanisms of neurotoxicity. Clin Neurosci Res 2001; 1: 407–18.
Shaw CA, Höglinger GU . Neurodegenerative diseases: neurotoxins as sufficient etiologic agents? Neuromolecular Med 2008; 10: 1–9.
Depboylu C, Schorlemmer K, Klietz M, Oertel WH, Weihe E, Höglinger GU, et al. Upregulation of microglial C1q expression has no effects on nigrostriatal dopaminergic injury in the MPTP mouse model of Parkinson disease. J Neuroimmunol 2011; 236: 39–46.
Ros-Bernal F, Hunot S, Herrero MT, Parnadeau S, Corvol JC, Lu L, et al. Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc Nati Acad Sci U S A 2011; 108: 6632–7.
Liu J, Wang MW, Gu P, Ma QY, Wang YY, Geng Y, et al. Microglial activation and age-related dopaminergic neurodegeneration in MPTP-treated SAMP8 mice. Brain Res 2010; 1345: 213–20.
Lee KW, Zhao X, Im JY, Grosso H, Jang WH, Chan TW, et al. Apoptosis signal-regulating kinase 1 mediates MPTP toxicity and regulates glial activation. PLoS One 2012; 7: e29935.
Phani S, Loike JD, Przedborski S . Neurodegeneration and inflammation in Parkinson's disease. Parkinsonism Relat Disord 2012; 18: S207–9.
Przedborski S . Inflammation and Parkinson's disease pathogenesis. Mov Disord 2010; 25: S55–7.
Khasnavis S, Jana A, Roy A, Mazumder M, Bhushan B, Wood T, et al. Suppression of nuclear factor-κB activation and inflammation in microglia by physically modified saline. J Biol Chem 2012; 287: 29529–42.
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH . Mechanisms underlying inflammation in neurodegeneration. Cell 2010; 140: 918–34.
Amor S, Puentes F, Baker D, Van Der Valk P . Inflammation in neurodegenerative diseases. Immunology 2010; 129: 154–69.
Whitton P . Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J pharmacol 2007; 150: 963–76.
Tansey MG, Goldberg MS . Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 2010; 37: 510–8.
Lim HJ, Li H, Kim JY . Quercetin derivatives from Siegesbeckia glabrescens inhibit the expression of COX-2 through the suppression of NF-κB activation in microglia. Biomal Ther 2011; 19: 27–32.
McNamara FN, Randall A, Gunthorpe MJ . Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br J pharmacol 2005; 144: 781–90.
Patacchini R, Maggi CA, Meli A . Capsaicin-like activity of some natural pungent substances on peripheral endings of visceral primary afferents. Naunyn Schmiedebergs Arch Pharmacol 1990; 342: 72–7.
Waggas AM . Neuroprotective evaluation of extract of ginger (Zingiber officinale) root in monosodium glutamate-induced toxicity in different brain areas male albino rats. Pak J Biol Sci 2009; 12: 201–12.
Subramanian U, Poongavanam S, Vanisree A . Studies on the neuroprotective role of Piper longum in C6 glioma induced rats. Invest New Drugs 2010; 28: 615–23.
Acknowledgements
This research was supported by Bio-industry Technology Development Program, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea (112136).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, G., Kim, H., Ju, M. et al. 6-Shogaol, an active compound of ginger, protects dopaminergic neurons in Parkinson's disease models via anti-neuroinflammation. Acta Pharmacol Sin 34, 1131–1139 (2013). https://doi.org/10.1038/aps.2013.57
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.57
Keywords
This article is cited by
-
Multivariate analysis of original identification and chemical markers exploration of Chinese ginger
Food Science and Biotechnology (2023)