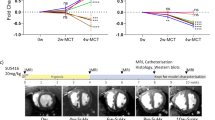
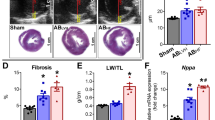
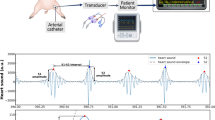
Abstract
Aim:
To study the effects of hydrogen sulfide (H2S) on the left ventricular expression of MMP-8, MMP-13, and TIMP-1 in a rat model of congenital heart disease.
Methods:
Male SD rats underwent abdominal aorta-inferior vena cava shunt operation. H2S donor NaHS (56 μmol·kg−1·d−1, ip) was injected from the next day for 8 weeks. At 8 weeks, the hemodynamic parameters, including the left ventricular systolic pressure (LVSP), the left ventricular peak rate of contraction and relaxation (LV±dp/dtmax) and the left ventricular end diastolic pressure (LVEDP) were measured. The left ventricular tissues were dissected out, and hydroxyproline and collagen I contents were detected with ELISA. The expression of MMP-8, MMP-13, and a tissue inhibitor of metalloproteinase-1 (TIMP-1) in the tissues was measured using real-time PCR, Western blotting, and immunohistochemistry, respectively.
Results:
The shunt operation markedly reduced LVSP and LV±dp/dtmax, increased LVEDP, hydroxyproline and collagen I contents, as well as the mRNA and protein levels of MMP-8, MMP-13, and TIMP-1 in the left ventricles. Chronic treatment of the shunt operation rats with NaHS effectively prevented the abnormalities in the hemodynamic parameters, hydroxyproline and collagen I contents, and the mRNA and protein levels of MMP-13 and TIMP-1 in the left ventricles. NaHS also prevented the increase of MMP-8 protein expression, but did not affect the increase of mRNA level of MMP-8 in the shunt operation rats.
Conclusion:
H2S suppresses protein and mRNA expression of MMP-8, MMP-13, and TIMP-1 in rats with cardiac volume overload, which may be contributed to the amelioration of ventricular structural remodeling and cardiac function.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Masutani S, Taketazu M, Ishido H, Iwamoto Y, Yoshiba S, Matsunaga T, et al. Effects of age on hemodynamic changes after transcatheter closure of atrial septal defect: importance of ventricular diastolic function. Heart Vessels 2012; 27: 71–8.
Liu YH, Yan CD, Bian JS . Hydrogen sulfide: a novel signaling molecule in the vascular system. J Cardiovasc Pharmacol 2011; 58: 560–9.
Liu YH, Lu M, Hu LF, Wong PT, Webb GD, Bian JS . Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signal 2012; 17: 141–85.
Muellner MK, Schreier SM, Laggner H, Hermann M, Esterbauer H, Exner M, et al. Hydrogen sulfide destroys lipid hydroperoxides in oxidized LDL. Biochem J 2009; 420: 277–81.
Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM . Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol 2008; 587: 1–7.
Su YW, Ding YG, Zhang CY, Zhang QY, Qi JG, Tang CS, et al. Changes hydrogen sulfide in plasma of children with congenital heart disease. J Appl Clin Pediatr 2005; 7: 632–4. Chinese.
Johansen D, Ytrehus K, Baxter GF . Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury evidence for a role of K-ATP channels. Basic Res Cardiol 2006; 101: 53–60.
Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, et al. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol 2007; 102: 261–8.
Li XH, Zhang CY, Zhang T . Sodium hydrosulfide improves cardiac functions and structures in rats with chronic heart failure. Zhonghua Yi Xue Za Zhi 2011; 91: 3044–9. Chinese.
Visse R, Nagase H . Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003; 92: 827–39.
Gunja-Smith Z, Morales AR, Romanelli R, Woessner JF . Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol 1996; 148: 1639–48.
Spinale FG, Coker ML, Bond BR, Zellner JL . Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovas Res 2000; 46: 225–38.
Peterson JT, Hallak H, Johnson L, Li H, O'Brien PM, Sliskovic DR, et al. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation 2001; 103: 2303–9.
Brew K, Nagase H . The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim Biophys Acta 2010; 1803: 55–71.
Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 2003; 284: H364–71.
Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, et al. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol 2005; 288: H149–58.
Chancey AL, Brower GL, Peterson JT, Janicki JS . Effects of matrix metalloproteinase inhibition on ventricular remodeling due to volume overload. Circulation 2002; 105: 1983–8.
Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ 3rd, Spinale FG . Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation 1998; 97: 1708–15.
Li H, Simon H, Bocan TM, Peterson JT . MMP/TIMP expression in spontaneously hypertensive heart failure rats: the effect of ACE- and MMP-inhibition. Cardiovasc Res 2000; 46: 298–306.
Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 2001; 104: 826–31.
Ocampo C, Ingram P, Ilbawi M, Arcilla R, Gupta M . Revisiting the surgical creation of volume load by aorto-caval shunt in rats. Mol Cell Biochem 2003; 251: 139–43.
Yan H, Du J, Tang C . The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun 2004; 313: 22–7.
Wang XL, Wang Q, Wei G, Zhu YZ . Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: a mechanism through cardiac mitochondrial protection. Biosci Rep 2011; 31: 87–98.
Sadowski SL . Congenital cardiac disease in the newborn infant: past, present, and future. Crit Care Nurs Clin North Am 2009; 21: 37–48.
Wang X, Ren B, Liu S, Sentex E, Tappia PS, Dhalla NS . Characterization of cardiac hypertrophy and heart failure due to volume overload in the rat. J Appl Physiol 2003; 94: 752–63.
Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, et al. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 2007; 293: H2093–100.
Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, D'Armiento J . Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest 2000; 106: 857–66.
George J, Patal S, Wexler D, Roth A, Sheps D, Keren G . Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am Heart J 2005; 150: 484–7.
Mishra PK, Tyagi N, Sen U, Givvimani S, Tyagi SC . H2S ameliorates oxidative and proteolytic stresses and protects the heart against adverse remodeling in chronic heart failure. Am J Physiol Heart Circ Physiol 2010; 298: H451–6.
Vincenti MP, Coon CI, Brinckerhoff CE . Nuclear factor kappaB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1beta-stimulated synovial fibroblasts. Arthritis Rheum 1998; 41: 1987–94.
Li YY, McTiernan CF, Feldman AM . Proinflammatory cytokines regulate tissue inhibitors of metalloproteinases and disintegrin metalloproteinase in cardiac cells. Cardiovasc Res 1999; 42: 162–72.
Yamamoto Y, Osanai T, Nishizaki F, Sukekawa T, Izumiyama K, Sagara S, et al. Matrix metalloprotein-9 activation under cell-to-cell interaction between endothelial cells and monocytes: possible role of hypoxia and tumor necrosis factor-α. Heart Vessels 2012; 27: 624–33.
Mossiat C, Demougeot C, Priqent-Tessier A, Bertrand N, Garnier P, Beley A, et al. Effects of iNOS-related NO on hearts exposed to liposoluble iron. Free Radic Res 2003; 37: 749–56.
Kimura Y, Kimura H . Hydrogen sulfide protects neurons from oxidative stress. FASEB J 2004; 18: 1165–7.
Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, et al. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology 2007; 241: 11–8.
Sen U, Basu P, Abe OA, Giwimani S, Tyagi N, Metreveli N, et al. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol 2009; 297: F410–9.
Bagatini MD, Martins CC, Battisti V, Gasparetto D, da Rosa CS, Spanevello RM, et al. Oxidative stress versus antioxidant defenses in patients with acute myocardial infarction. Heart Vessels 2011; 26: 55–63.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No 30872787), Beijing Outstanding Talents Training Program (20081D0303200107) and Science Foundation for High-Level Medical Talents of Beijing Health System (2011-3-067).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Cy., Li, Xh., Zhang, T. et al. Hydrogen sulfide suppresses the expression of MMP-8, MMP-13, and TIMP-1 in left ventricles of rats with cardiac volume overload. Acta Pharmacol Sin 34, 1301–1309 (2013). https://doi.org/10.1038/aps.2013.84
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.84