Abstract
Aim:
Forkhead box M1 (FoxM1) is a transcription factor that plays important roles in the pathogenesis and progression of human cancers, including hepatocellular carcinoma (HCC). The aim of this study was to examine the involvement of FoxM1 in the anti-cancer action of sorafenib, a multikinase inhibitor, in human HCC cells.
Methods:
HCC cell lines HepG2 and HuH-7 were tested. Cell viability was examined using MTT assay and cell invasion was determined with Transwell migration assay. The relevant mRNA expression was determined with RT-PCR, and the proteins were detected using Western blotting and immunofluorescence assays. RNA interference was used to modify the expression of p53 and FoxM1. HuH-7 cell line xenograft mice were used for in vivo study, which were treated with sorafenib (40 mg/kg, po) daily for 3 weeks.
Results:
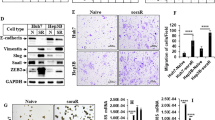
Sorafenib (2–20 μmol/L) inhibited the proliferation of the cells in dose- and time-dependent manners with an IC50 value of nearly 6 μmol/L at 48 h. Sorafenib (6 μmol/L) markedly suppressed the cell invasion. Furthermore, sorafenib (2−6 μmol/L) dose-dependently decreased the expression of FoxM1, MMP-2, and Ki-67, and up-regulated that of p53 in the cells. Silencing p53 abolished the decrease of FoxM1 and increase of p53 in sorafenib-treated cells. Silencing FoxM1 significantly reduced the expression of MMP-2 and Ki-67, and enhanced the anti-proliferation action of sorafenib in the cells, whereas overexpression of FoxM1 increased the expression of MMP-2 and Ki-67, and abrogated the anti-proliferation action of sorafenib. In the xenograft mice, sorafenib administration decreased the tumor growth by 40%, and markedly increased the expression of p53, and decreased the expression of FoxM1, MMP-2, and Ki-67 in tumor tissues.
Conclusion:
Sorafenib inhibits HCC proliferation and invasion by inhibiting MMP-2 and Ki-67 expression due to up-regulation of P53 and suppressing FoxM1.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kim DY, Paik YH, Ahn SH, Youn YJ, Choi JW, Kim JK, et al. PIVKA-II is a useful tumor marker for recurrent hepatocellular carcinoma after surgical resection. Oncology 2007; 72: 52–7.
Scotto KW, Biedler JL, Melera PW . Amplification and expression of genes associated with multidrug resistance in mammalian cells. Science 1986; 232: 751–5.
Bruix J, Sherman M . American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–24.
Lang L . FDA approves sorafenib for patients with inoperable Liver Diseases. cancer. Gastroenterology 2008; 134: 379.
Hahn O, Stadler W . Sorafenib. Curr Opin Oncol 2006; 18: 615–21.
Rini BI . Sorafenib. Expert Opin Pharmacother 2006; 7: 453–61.
Wilhelm S, Chien DS . BAY 43-9006: preclinical data. Curr Pharm Des 2002; 8: 2255–7.
Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene 2005; 24: 6861–9.
Ho C, Wang C, Mattu S, Destefanis G, Ladu S, Delogu S, et al. AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways. Hepatology 2012; 55: 833–45.
Wu QF, Liu C, Tai MH, Liu D, Lei L, Wang RT, et al. Knockdown of FoxM1 by siRNA interference decreases cell proliferation, induces cell cycle arrest and inhibits cell invasion in MHCC-97H cells in vitro. Acta Pharmacol Sin 2010; 31: 361–6.
Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol 2005; 25: 10875–94.
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH . Forkhead box M1 transcription factor: a novel target for cancer therapy. Cancer Treat Rev 2010; 36: 151–6.
Laoukili J, Stahl M, Medema RH . FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta 2007; 1775: 92–102.
Ma RY, Tong TH, Leung WY, Yao KM . Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1. In: Transcription Factors: Springer, 2010; 113–23.
Balli D, Zhang Y, Snyder J, Kalinichenko VV, Kalin TV . Endothelial cell-specific deletion of transcription factor FoxM1 increases urethane-induced lung carcinogenesis. Cancer Res 2011; 71: 40–50.
Zhang Y, Zhang N, Dai B, Liu M, Sawaya R, Xie K, et al. FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells. Cancer Res 2008; 68: 8733–42.
Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH . Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res 2007; 67: 8293–300.
Tan Y, Raychaudhuri P, Costa RH . Chk2 mediates stabilization of the FoxM1 transcription factor to stimulate expression of DNA repair genes. Mol Cell Biol 2007; 27: 1007–16.
Myatt SS, Lam EW-F . The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007; 7: 847–59.
Kwok JMM, Peck B, Monteiro LJ, Schwenen HDC, Millour J, Coombes RC, et al. FOXM1 confers acquired cisplatin resistance in breast cancer cells. Mol Cancer Res 2010; 8: 24–34.
Carr JR, Park HJ, Wang Z, Kiefer MM, Raychaudhuri P . FoxM1 mediates resistance to herceptin and paclitaxel. Cancer Res 2010; 70: 5054–63.
Qu K, Xu X, Liu C, Wu Q, Wei J, Meng F, et al. Negative regulation of transcription factor FoxM1 by p53 enhances oxaloplatin-induced senescence in hepatocellular carcinoma. Cancer Lett 2012; 331: 105–14.
Sun H, Teng M, Liu J, Jin D, Wu J, Yan D, et al. FOXM1 expression predicts the prognosis in hepatocellular carcinoma patients after orthotopic liver transplantation combined with the Milan criteria. Cancer Lett 2011; 306: 214–22.
Limin X, Wenjie H, Dean T, Hongwu Z, Yongguo Z, Hao H, et al. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J Hepatol 2012; 57: 600–12.
Bhat UG, Halasi M, Gartel AL . Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PLoS One 2009; 4: e5592.
McGovern UB, Francis RE, Peck B, Guest SK, Wang J, Myatt SS, et al. Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast cancer. Mol Cancer Ther 2009; 8: 582–91.
Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, et al. Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells. J Cell Biochem 2011; 112: 78–88.
Crawford LJ, Walker B, Irvine AE . Proteasome inhibitors in cancer therapy. J Cell Commun Signal 2011; 5: 101–10.
Feng YX, Wang T, Deng YZ, Yang P, Li JJ, Guan DX, et al. Sorafenib suppresses postsurgical recurrence and metastasis of hepatocellular carcinoma in an orthotopic mouse model. Hepatology 2011; 53: 483–92.
de Olano N, Koo CY, Monteiro LJ, Pinto PH, Gomes AR, Aligue R, et al. The p38 MAPK–MK2 Axis regulates E2F1 and FOXM1 expression after epirubicin treatment. Mol Cancer Res 2012; 10: 1189–202.
Costa RH . FoxM1 dances with mitosis. Nat Cell Biol 2005; 7: 108–10.
Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, et al. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol 2008; 10: 1076–82.
Beier R, Bürgin A, Kiermaier A, Fero M, Karsunky H, Saffrich R, et al. Induction of cyclin E-cdk2 kinase activity, E2F-dependent transcription and cell growth by Myc are genetically separable events. EMBO J 2000; 19: 5813–23.
Leung KC, Hsin MKY, Chan JSY, Yip JHY, Li M, Leung B, et al. Inhibition of thromboxane synthase induces lung cancer cell death via increasing the nuclear p27. Exper Cell Res 2009; 315: 2974–81.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34.
Kong HH, Sibaud V, Chanco Turner ML, Fojo T, Hornyak TJ, et al. Sorafenib-induced eruptive melanocytic lesions. Arch Dermatol 2008; 144: 820–2.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No 30872482 and No 81072051) and the project of Innovative Research Team for Key Science and Technology in Xi'an Jiaotong University (No 2003KCJ-23).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, Jc., Meng, Fd., Qu, K. et al. Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1. Acta Pharmacol Sin 36, 241–251 (2015). https://doi.org/10.1038/aps.2014.122
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2014.122
Keywords
This article is cited by
-
Inhibition of Dickkopf-1 enhances the anti-tumor efficacy of sorafenib via inhibition of the PI3K/Akt and Wnt/β-catenin pathways in hepatocellular carcinoma
Cell Communication and Signaling (2023)
-
p53 in ferroptosis regulation: the new weapon for the old guardian
Cell Death & Differentiation (2022)
-
The effects of Sorafenib and Natural killer cell co-injection in combinational treatment of hepatocellular carcinoma; an in vivo approach
Pharmacological Reports (2022)
-
Histone deacetylase inhibitor resminostat in combination with sorafenib counteracts platelet-mediated pro-tumoral effects in hepatocellular carcinoma
Scientific Reports (2021)
-
Taurine Upregulates miRNA-122-5p Expression and Suppresses the Metabolizing Enzymes of Glycolytic Pathway in Hepatocellular Carcinoma
Molecular Biology Reports (2021)