Abstract
Aim:
To investigate the effects of liraglutide, a glucagon-like peptide-1 (GLP-1) receptor activator, on body weight and waist circumference in Chinese overweight and obese type 2 diabetic patients.
Methods:
A total of 328 Chinese overweight and obese type 2 diabetic patients were included in this multi-center, open-labeled and self-controlled clinical study. The patients were subcutaneously injected with liraglutide once daily for 24 weeks as add-on therapy to their previous hypoglycemic treatments. Statistical analyses were performed using SPSS software package version 11.5 for Windows.
Results:
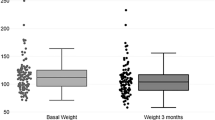
Liraglutide treatment caused significant reduction of the mean body weight (from 86.61±14.09 to 79.10±13.55 kg) and waist circumference (from 101.81±13.96 to 94.29±14.17 cm), resulting in body weight lose of 5%–10% in 43.67% patients, and body weight loss above 10% in 34.06% patients, who had significant lower plasma creatinine levels. Baseline waist circumference, BMI and HOMA-IR were independently correlated with the body weight loss. Furthermore, liraglutide treatment significantly decreased HbA1c levels (from 8.66%±2.17% to 6.92%±0.95%) with HbA1c<7.0% in 35.37% patients, who had a significantly lower baseline level of HbA1c, but higher baseline levels of C peptide and glucagon. Moreover, liraglutide treatment resulted in greater body weight loss in patients with a long duration of diabetes, and better glycemic control in patients with a short duration of diabetes.
Conclusion:
Liraglutide significantly reduces body weight and waist circumference in Chinese overweight and obese type 2 diabetic patients. Patients with apparent visceral obesity, insulin resistance and a long duration of diabetes may have greater body weight loss; whereas patients with high insulin-secreting ability, hyperglucagonemia, and short-duration diabetes may obtain better glycemic control with liraglutide.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–43.
UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–53.
Green J, Feinglos M . Update on type 2 diabetes mellitus: understanding changes in the diabetes treatment paradigm. Int J Clin Pract 2007; 61 (Suppl 154): 3–11.
Holst JJ . The physiology of glucagon-like peptide 1. Physiol Rev 2007; 87: 1409–39.
Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ . Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000; 43: 1664–9.
Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M . The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 2002; 45: 195–202.
Nauck M, Frid A, Hermansen K, Thomsen AB, During M, Shah N, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab 2013; 15: 204–12.
Yang W, Chen L, Ji Q, Liu X, Ma J, Tandon N, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial. Diabetes Obes Metab 2011; 13: 81–8.
Ostoft SH, Bagger JI, Hansen T, Pedersen O, Faber J, Holst JJ, et al. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care 2014; 37: 1797–805.
Suzuki D, Toyoda M, Kimura M, Miyauchi M, Yamamoto N, Sato H, et al. Effects of liraglutide, a human glucagon-like peptide-1 analogue, on body weight, body fat area and body fat-related markers in patients with type 2 diabetes mellitus. Intern Med 2013; 52: 1029–34.
Jensterle Sever M, Kocjan T, Pfeifer M, Kravos NA, Janez A . Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J Endocrinol 2014; 170: 451–9.
Eguchi Y, Kitajima Y, Hyogo H, Takahashi H, Kojima M, Ono M, et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res 2014 May 4. doi: 10.1111/hepr.12351. [Epub ahead of print]
de Wit HM, Vervoort GM, Jansen HJ, de Grauw WJ, de Galan BE, Tack CJ . Liraglutide reverses pronounced insulin-associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT). Diabetologia 2014 Jun 20. [Epub ahead of print]
Li CJ, Li J, Zhang QM, Lv L, Chen R, Lv CF, et al. Efficacy and safety comparison between liraglutide as add-on therapy to insulin and insulin dose-increase in Chinese subjects with poorly controlled type 2 diabetes and abdominal obesity. Cardiovasc Diabetol 2012; 11: 142.
Li CJ, Yu Q, Yu P, Yu TL, Zhang QM, Lu S, Yu DM . Changes in liraglutide-induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovasc Diabetol 2014; 13: 36.
Bei-Fan Z . Cooperative meta-analysis group of working group on obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac J Clin Nutr 2002; 11: S685–S693.
Inzucchi SE, Bergenstal RM, Buse JB . Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetologia 2012; 55: 1577–96.
Niswender K, Pi-Sunyer X, Buse J . Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab 2013; 15: 42–54.
Zander M, Madsbad S, Madsen JL, Holst JJ . Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002; 359: 824–30.
van Genugten RE, Moller-Goede DL, van Raalte DH, Diamant M . Extra-pancreatic effects of incretin-based therapies: potential benefit for cardiovascular-risk management in type 2 diabetes. Diabetes Obes Metab 2013; 15: 593–606.
Bode BW, Testa MA, Magwire M, Hale PM, Hammer M, Blonde L, et al. Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 604–12.
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide effect and action in diabetes 5 (LEAD-5) met+SU study group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–55.
Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374: 39–47.
Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26: 268–78.
Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009; 32: 1224–30.
Klein S, Sheard NF, Pi-Sunyer X . Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care 2004; 27: 2067–73.
Yajnik CS, Yudkin JS . The Y-Y paradox. Lancet 2004; 363: 163.
Dagenais GR, Yi Q, Mann JF . Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J 2005; 149: 54–60.
Fadini GP, Simioni N, Frison V, Dal Pos M, Bettio M, Rocchini P, et al. Independent glucose and weight-reducing effects of liraglutide in a real-world population of type 2 diabetic outpatients. Acta Diabetol 2013; 50: 943–9.
Park J, Mehrotra R, Rhee CM, Molnar MZ, Lukowsky LR, Patel SS, et al. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol Dial Transplant 2013; 28: 2146–55.
Mamtani M, Kulkarni H, Dyer TD, Almasy L, Mahaney MC, Duggirala R, et al. Waist circumference independently associates with the risk of insulin resistance and type 2 diabetes in mexican american families. PLoS One 2013; 8: e 59153.
Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, Düring M, et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab 2009; 11: 1163–72.
Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373: 473–81.
Heritier SR, Gebski VJ, Keech AC . Inclusion of patients in clinical trial analysis: the intention-to-treat principle. Med J Aust 2003; 179: 438–40.
Hollis S, Campbell F . What is meant by intention to treat analysis. Survey of published randomised controlled trials? BMJ 1999; 319: 670–4.
Acknowledgements
We acknowledge the assistance of investigators and all subjects for their participation in this study. This work was supported by the National Natural Science Foundation of China (No 81200612) and the Tianjin City High School Science & Technology Fund Planning Project (No 20102217). We also acknowledge Allen Clermont from the Joslin Diabetes Center of Harvard Medical School for assistance in the writing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, P., Yu, Dm., Chen, Lm. et al. Liraglutide reduces the body weight and waist circumference in Chinese overweight and obese type 2 diabetic patients. Acta Pharmacol Sin 36, 200–208 (2015). https://doi.org/10.1038/aps.2014.136
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2014.136
Keywords
This article is cited by
-
Treatment effect heterogeneity following type 2 diabetes treatment with GLP1-receptor agonists and SGLT2-inhibitors: a systematic review
Communications Medicine (2023)
-
Glucagon-like peptide-1 mimetics, optimal for Asian type 2 diabetes patients with and without overweight/obesity: meta-analysis of randomized controlled trials
Scientific Reports (2017)
-
Study on the adult physique with the Heath-Carter anthropometric somatotype in the Han of Xi’an, China
Anatomical Science International (2016)