Abstract
Aim:
Lapatinib is a dual inhibitor of EGFR and human epidermal growth factor receptor 2 (HER2), and used to treat advanced breast cancer. To overcome its poor water solubility, we constructed lapatinib-incorporated lipoprotein-like nanoparticles (LTNPs), and evaluated the particle characteristics and possible anti-breast cancer mechanisms.
Methods:
LTNPs (lapatinib bound to albumin as a core, and egg yolk lecithin forming a lipid corona) were prepared. The particle characteristics were investigated using transmission electron microscopy (TEM) and atomic force microscopy (AFM). The uptake and subcellular localization of LTNPs, as well as the effects of LTNPs on cell cycle were examined in BT-474 human breast cancer cells in vitro. Mice bearing BT-474 subcutaneous xenograft were intravenously injected with coumarin-6 loaded LTNPs (30 mg/kg) to study the targeting mechanisms in vivo.
Results:
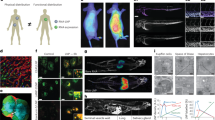
The LTNPs particles were generally spherical but flexible under TEM and AFM, and approximately 62.1 nm in size with a zeta potential of 22.80 mV. In BT-474 cells, uptake of LTNPs was mediated by endosomes through energy-dependent endocytosis involving clathrin-dependent pinocytosis and macropinocytosis, and they could effectively escape from endosomes to the cytoplasm. Treatment of BT-474 cells with LTNPs (20 μg/mL) induced a significant cell arrest at G0/G1 phase compared with the same concentration of lapatinib suspension. In mice bearing BT-474 xenograft, intravenously injected LTNPs was found to target and accumulate in tumors, and colocalized with HER2 and SPRAC (secreted protein, acidic and rich in cysteine).
Conclusion:
LTNPs can be taken up into breast cancer cells through specific pathways in vitro, and targeted to breast cancer xenograft in vivo via enhanced permeability and retention effect and SPARC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Moreira C, Kaklamani V . Lapatinib and breast cancer: current indications and outlook for the future. Expert Rev Anticancer Ther 2010; 10: 1171–82.
Medina PJ, Goodin S . Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther 2008; 30: 1426–47.
Burstein HJ . The distinctive nature of HER2-positive breast cancers. N Engl J Med 2005; 353: 1652–54.
LaBonte MJ, Wilson PM, Fazzone W, Russell J, Louie SG, El-Khoueiry A, et al. The dual EGFR/HER2 inhibitor lapatinib synergistically enhances the antitumor activity of the histone deacetylase inhibitor panobinostat in colorectal cancer models. Cancer Res 2011; 71: 3635–48.
Gao H, Yang Z, Cao S, Xi Z, Zhang S, Pang Z, et al. Behavior and anti-glioma effect of lapatinib-incorporated lipoprotein-like nanoparticles. Nanotechnology 2012; 23: 435101.
Olaussen KA, Commo F, Tailler M, Lacroix L, Vitale I, Raza SQ, et al. Synergistic proapoptotic effects of the two tyrosine kinase inhibitors pazopanib and lapatinib on multiple carcinoma cell lines. Oncogene 2009; 28: 4249–60.
Gao H, Cao S, Chen C, Cao S, Yang Z, Pang Z, et al. Incorporation of lapatinib into lipoprotein-like nanoparticles with enhanced water solubility and anti-tumor effect in breast cancer. Nanomedicine (Lond) 2013; 8: 1429–42.
Heikkila JE, Vaha-Koskela MJ, Ruotsalainen JJ, Martikainen MW, Stanford MM, McCart JA, et al. Intravenously administered alphavirus vector VA7 eradicates orthotopic human glioma xenografts in nude mice. PLoS One 2010; 5: e8603.
Gao H, Pan S, Yang Z, Cao S, Chen C, Jiang X, et al. A cascade targeting strategy for brain neuroglial cells employing nanoparticles modified with angiopep-2 peptide and EGFP-EGF1 protein. Biomaterials 2011; 32: 8669–75.
Gao H, Wang Y, Chen C, Chen J, We Y, Cao S, et al. Incorporation of lapatinib into core-shell nanoparticles improves both the solubility and anti-glioma effects of the drug. Int J Pharm 2014; 461: 478–88.
Liu J, Shapiro JI . Endocytosis and signal transduction: basic science update. Biol Res Nurs 2003; 5: 117–28.
Gao H, Yang Z, Zhang S, Cao S, Shen S, Pang Z, et al. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci Rep 2013; 3: 2534.
Lu W, Sun Q, Wan J, She Z, Jiang XG . Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res 2006; 66: 11878–87.
Zhang LM, Zhang JN, Yang SW, Dong C, Fang DD, Li J, et al. Expression of HER2 in glioma cell lines A172, U251, U87 and SHG-44 and its significance. Chin J Neurosurg Dis Res 2011; 10: 151–53.
Barenholz Y . Doxil®—The first FDA-approved nano-drug: Lessons learned. J Control Release 2012; 160: 117–34.
Gao H, Pang Z, Pan S, Cao S, Yang Z, Chen C, et al. Anti-glioma effect and safety of docetaxel-loaded nanoemulsion. Arch Pharm Res 2012; 35: 333–41.
Gao H, Pang Z, Jiang X . Targeted delivery of nano-therapeutics for major disorders of the central nervous system. Pharm Res 2013; 30: 2485–98.
Mahmoudi M, Azadmanesh K, Shokrgozar MA, Journeay WS, Laurent S . Effect of nanoparticles on the cell life cycle. Chem Rev 2011; 111: 3407–32.
Maeda H . SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv Drug Deliv Rev 2001; 46: 169–85.
Matsumura Y, Maeda H . A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986; 46: 6387–92.
Torchilin VP . Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol 2010; (197): 3–53.
Fang J, Sawa T, Maeda H . Factors and mechanism of “EPR” effect and the enhanced antitumor effects of macromolecular drugs including SMANCS. Adv Exp Med Biol 2003; 519: 29–49.
Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A 1998; 95: 4607–12.
Acknowledgements
The work was funded by the National Natural Science Foundation of China (81373337) and the Sichuan University Starting Foundation for Young Teachers (2014SCU11044).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Zhang, S., Ruan, Sb. et al. Lapatinib-incorporated lipoprotein-like nanoparticles: preparation and a proposed breast cancer-targeting mechanism. Acta Pharmacol Sin 35, 846–852 (2014). https://doi.org/10.1038/aps.2014.26
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2014.26
Keywords
This article is cited by
-
Application of atomic force microscopy in cancer research
Journal of Nanobiotechnology (2018)
-
Co-delivery of docetaxel and silibinin using pH-sensitive micelles improves therapy of metastatic breast cancer
Acta Pharmacologica Sinica (2017)
-
Silibinin and indocyanine green-loaded nanoparticles inhibit the growth and metastasis of mammalian breast cancer cells in vitro
Acta Pharmacologica Sinica (2016)