Abstract
Aim:
Interleukin-22 (IL-22) exhibits both proinflammatory and anti-inflammatory properties in various biological processes. In this study we explored the effects of exogenous recombinant IL-22 (rIL-22) on cigarette smoke (CS)-induced airway inflammation in mice.
Methods:
Male C57BL/6 mice were divided into groups: (1) CS group exposed to tobacco smoke for 3 consecutive days, (2) rIL-22 group received rIL-22 (100 mg/kg, ip), and (3) CS plus rIL-22 group, received rIL-22 (100 mg/kg, ip) before the CS exposure. The airway resistance (Rn), lung morphology, inflammatory cells in the airways, and inflammatory cytokines and CXCR3 ligands in both bronchoalveolar lavage (BAL) fluids and lung tissues were analyzed.
Results:
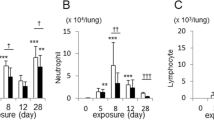
CS alone significantly elevated IL-22 level in the BAL fluid. Both CS and rIL-22 significantly augmented airway resistance, an influx of inflammatory cells into the airways and lung parenchyma, and significantly elevated levels of pro-inflammatory cytokines (TGFβ1 and IL-17A) and CXCR3 chemokines (particularly CXCL10) at the mRNA and/or protein levels. Furthermore, the effects of rIL-22 on airway resistance and inflammation were synergistic with those of CS, as demonstrated by a further increased Rn value, infiltration of greater numbers of inflammatory cells into the lung, higher levels of inflammatory cytokines and chemokines, and more severe pathological changes in CS plus rIL-22 group as compared to those in CS group.
Conclusion:
Exogenous rIL-22 exacerbates the airway inflammatory responses to CS exposure in part by inducing expression of several proinflammatory cytokines and CXCR3 ligands.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zenewicz LA, Flavell RA . Recent advances in IL-22 biology. Int Immunol 2011; 23: 159–63.
Nembrini C, Marsland BJ, Kopf M . IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol 2009; 123: 986–94.
Ouyang W, Valdez P . IL-22 in mucosal immunity. Mucosal Immunol 2008; 1: 335–8.
Rutz S, Eidenschenk C, Ouyang W . IL-22, not simply a Th17 cytokine. Immunol Rev 2013; 252: 116–32.
Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG . Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011; 29: 71–109.
Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem 2012; 287: 8816–29.
Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C . IL-17 and IL-22: siblings, not twins. Trends Immunol 2010; 31: 354–61.
Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W, et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A 2013; 110: 12768–73.
Sonnenberg GF, Fouser LA, Artis D . Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 2011; 12: 383–90.
Sonnenberg GF, Fouser LA, Artis D . Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol 2010; 107: 1–29.
Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, et al. Dual Role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med 2011; 183: 1153–63.
Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, et al. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol 2011; 44: 369–76.
Aujla SJ, Alcorn JF . TH17 cells in asthma and inflammation. Biochim Biophys Acta 2011; 1810: 1066–79.
Liang M, Wang J, Chu H, Zhu X, He H, Liu Q, et al. Interleukin-22 inhibits bleomycin-induced pulmonary fibrosis. Mediators Inflamm 2013; 2013: 209179.
Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D . Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med 2010; 207: 1293–305.
Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J Allergy Clin Immunol 2011; 128: 1067–76.
Pennino D, Bhavsar PK, Effner R, Avitabile S, Venn P, Quaranta M, et al. IL-22 suppresses IFN-gamma-mediated lung inflammation in asthmatic patients. J Allergy Clin Immunol 2013; 131: 562–70.
Muhl H . Pro-inflammatory signaling by IL-10 and IL-22: Bad habit stirred up by interferons? Front Immunol 2013; 4: 18.
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007; 445: 648–51.
Hirose K, Takahashi K, Nakajima H . Roles of IL-22 in allergic airway inflammation. J Allergy (Cairo) 2013; 2013: 260518.
Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–65.
Smyth LJ, Starkey C, Vestbo J, Singh D . CD4-regulatory cells in COPD patients. Chest 2007; 132: 156–63.
Smyth LJ, Starkey C, Gordon FS, Vestbo J, Singh D . CD8 chemokine receptors in chronic obstructive pulmonary disease. Clin Exp Immunol 2008; 154: 56–63.
Brusselle GG, Joos GF, Bracke KR . New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011; 378: 1015–26.
Zhang L, Cheng Z, Liu W, Wu K . Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. COPD 2013; 10: 459–65.
Paats MS, Bergen IM, Hoogsteden HC, van der Eerden MM, Hendriks RW . Systemic CD4+ and CD8+ T-cell cytokine profiles correlate with GOLD stage in stable COPD. Eur Respir J 2012; 40: 330–7.
Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol 2009; 157: 316–24.
Nie L, Xiang R, Zhou W, Lu B, Cheng D, Gao J . Attenuation of acute lung inflammation induced by cigarette smoke in CXCR3 knockout mice. Respir Res 2008; 9: 82.
Nie L, Xiang RL, Liu Y, Zhou WX, Jiang L, Lu B, et al. Acute pulmonary inflammation is inhibited in CXCR3 knockout mice after short-term cigarette smoke exposure. Acta Pharmacol Sin 2008; 29: 1432–9.
Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, et al. IL-22 induces an acute-phase response. J Immunol 2010; 185: 5531–8.
Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 2012; 143: 765–76.
Zhu Y, Liu Y, Zhou W, Xiang R, Jiang L, Huang K, et al. A prostacyclin analogue, iloprost, protects from bleomycin-induced pulmonary fibrosis in mice. Respir Res 2010; 11: 34.
Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H . Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol 2009; 10: 864–71.
Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978; 298: 1277–81.
Jia YT, Wei W, Ma B, Xu Y, Liu WJ, Wang Y, et al. Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. J Immunol 2007; 179: 7808–19.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–73.
Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, et al. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol 2013; 182: 1286–96.
Alcorn JF, Crowe CR, Kolls JK . TH17 cells in asthma and COPD. Annu Rev Physiol 2010; 72: 495–516.
Kitamura M, Kasai A . Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett 2007; 252: 184–94.
Torii K, Saito C, Furuhashi T, Nishioka A, Shintani Y, Kawashima K, et al. Tobacco smoke is related to Th17 generation with clinical implications for psoriasis patients. Exp Dermatol 2011; 20: 371–3.
D'Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F . Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med 2001; 164: 1266–75.
Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 165: 1404–9.
Kelsen SG, Aksoy MO, Georgy M, Hershman R, Ji R, Li X, et al. Lymphoid follicle cells in chronic obstructive pulmonary disease overexpress the chemokine receptor CXCR3. Am J Respir Crit Care Med 2009; 179: 799–805.
Laurence A, O'Shea JJ, Watford WT . Interleukin-22: a sheep in wolf's clothing. Nat Med 2008; 14: 247–9.
Jones CE, Chan K . Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol 2002; 26: 748–53.
Johnson JR, Nishioka M, Chakir J, Risse PA, Almaghlouth I, Bazarbashi AN, et al. IL-22 contributes to TGF-beta1-mediated epithelial-mesenchymal transition in asthmatic bronchial epithelial cells. Respir Res 2013; 14: 118.
Acknowledgements
This work is partly supported by National Natural Science Foundation of China (81170040 and 81470229), and National Science and Technology Pillar Program during the Twelfth Five-year Period 2012BAI05B00.
We are grateful for Prof Guan-liang SHAN's kind help in facilitating the whole experiment, and we thank the technicians at the Department of Pathology, Peking Union Medical College Hospital for help in generating the pathological slides and the staff of the Animal Center, Peking Union Medical College Hospital for caring for the animals. We thank Dr Xi-qiang YAN for his generosity in providing recombinant IL-22 and his helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Jr., Zhou, Wx., Huang, Kw. et al. Interleukin-22 exacerbates airway inflammation induced by short-term exposure to cigarette smoke in mice. Acta Pharmacol Sin 35, 1393–1401 (2014). https://doi.org/10.1038/aps.2014.91
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2014.91