Abstract
Aim:
Parkin has been shown to exert protective effects against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in different models of Parkinson disease. In the present study we investigated the molecular mechanisms underlying the neuroprotective action of parkin in vitro.
Methods:
HEK293, HeLa and PC12 cells were transfected with parkin, parkin mutants, p62 or si-p62. Protein expression and ubiquitination were assessed using immunoblot analysis. Immunoprecipitation assay was performed to identify the interaction between parkin and scaffold protein p62. PC12 and SH-SY5Y cells were treated with 6-OHDA (200 μmol/L), and cell apoptosis was detected using PI and Hoechst staining.
Results:
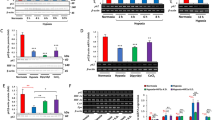
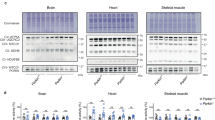
In HEK293 cells co-transfected with parkin and p62, parkin was co-immunoprecipitated with p62, and parkin overexpression increased p62 protein levels. In parkin-deficient HeLa cells, transfection with wild-type pakin, but not with ligase activity-deficient pakin mutants, significantly increased p62 levels, suggesting that parkin stabilized p62 through its E3 ligase activity. Transfection with parkin or p62 significantly repressed ERK1/2 phosphorylation in HeLa cells, but transfection with parkin did not repress ERK1/2 phosphorylation in p62-knockdown HeLa cells, suggesting that p62 was involved in parkin-induced inhibition on ERK1/2 phosphorylation. Overexpression of parkin or p62 significantly repressed 6-OHDA-induced ERK1/2 phosphorylation in PC12 cells, and parkin overexpression inhibited 6-OHDA-induced apoptosis in PC12 and SH-SY5Y cells.
Conclusion:
Parkin protects PC12 cells against 6-OHDA-induced apoptosis via ubiquitinating and stabilizing scaffold protein p62, and repressing ERK1/2 activation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Farrer MJ . Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet 2006; 7: 306–18.
Thomas B, Beal MF . Parkinson's disease. Hum Mol Genet 2007; 16: R183–94.
Dekker MC, Bonifati V, van Duijn CM . Parkinson's disease: piecing together a genetic jigsaw. Brain 2003; 126: 1722–33.
Bossy-Wetzel E, Schwarzenbacher R, Lipton SA . Molecular pathways to neurodegeneration. Nat Med 2004; 10 Suppl: S2–9.
Tan EK, Skipper LM . Pathogenic mutations in Parkinson disease. Hum Mutat 2007; 28: 641–53.
Mata IF, Lockhart PJ, Farrer MJ . Parkin genetics: one model for Parkinson's disease. Hum Mol Genet 2004; 13: R127–33.
Jiang H, Ren Y, Zhao J, Feng J . Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet 2004; 13: 1745–54.
Youle RJ, Narendra DP . Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011; 12: 9–14.
Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, et al. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem 2010; 285: 27879–90.
Narendra D, Tanaka A, Suen DF, Youle RJ . Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy 2009; 5: 706–8.
Manley S, Williams JA, Ding WX . Role of p62/SQSTM1 in liver physiology and pathogenesis. Exp Biol Med (Maywood) 2013; 238: 525–38.
Cavey JR, Ralston SH, Hocking LJ, Sheppard PW, Ciani B, Searle MS, et al. Loss of ubiquitin-binding associated with Paget's disease of bone p62 (SQSTM1) mutations. J Bone Miner Res 2005; 20: 619–24.
Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell 2009; 137: 1062–75.
Lee SJ, Pfluger PT, Kim JY, Nogueiras R, Duran A, Pages G, et al. A functional role for the p62-ERK1 axis in the control of energy homeostasis and adipogenesis. EMBO Rep 2010; 11: 226–32.
Rodriguez A, Duran A, Selloum M, Champy MF, Diez-Guerra FJ, Flores JM, et al. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab 2006; 3: 211–22.
Cagnol S, Chambard JC . ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J 2010; 277: 2–21.
Bost F, Aouadi M, Caron L, Even P, Belmonte N, Prot M, et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes 2005; 54: 402–11.
Lindgren N, Leak RK, Carlson KM, Smith AD, Zigmond MJ . Activation of the extracellular signal-regulated kinases 1 and 2 by glial cell line-derived neurotrophic factor and its relation to neuroprotection in a mouse model of Parkinson's disease. J Neurosci Res 2008; 86: 2039–49.
Wang XL, Xing GH, Hong B, Li XM, Zou Y, Zhang XJ, et al. Gastrodin prevents motor deficits and oxidative stress in the MPTP mouse model of Parkinson's disease: involvement of ERK1/2-Nrf2 signaling pathway. Life Sci 2014; 114: 77–85.
Lee E, Park HR, Ji ST, Lee Y, Lee J . Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson's disease model by downregulating the activations of nuclear factor-kappaB, ERK, and JNK. J Neurosci Res 2014; 92: 130–9.
Benskey MJ, Manfredsson FP, Lookingland KJ, Goudreau JL . The role of parkin in the differential susceptibility of tuberoinfundibular and nigrostriatal dopamine neurons to acute toxicant exposure. Neurotoxicology 2015; 46: 1–11.
Park HJ, Park KH, Shin KS, Lee MK . The roles of cyclic AMP-ERK-Bad signaling pathways on 6-hydroxydopamine-induced cell survival and death in PC12 cells. Toxicol In Vitro 2013; 27: 2233–41.
Santini E, Alcacer C, Cacciatore S, Heiman M, Herve D, Greengard P, et al. L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem 2009; 108: 621–33.
Reinhardt P, Schmid B, Burbulla LF, Schondorf DC, Wagner L, Glatza M, et al. Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell 2013; 12: 354–67.
Ren Y, Jiang H, Yang F, Nakaso K, Feng J . Parkin protects dopaminergic neurons against microtubule-depolymerizing toxins by attenuating microtubule-associated protein kinase activation. J Biol Chem 2009; 284: 4009–17.
Ren H, Fu K, Mu C, Li B, Wang D, Wang G . DJ-1, a cancer and Parkinson's disease associated protein, regulates autophagy through JNK pathway in cancer cells. Cancer Lett 2010; 297: 101–8.
Chen D, Gao F, Li B, Wang H, Xu Y, Zhu C, et al. Parkin mono-ubiquitinates Bcl-2 and regulates autophagy. J Biol Chem 2010; 285: 38214–23.
Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 2000; 25: 302–5.
Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 2010; 12: 119–31.
Denison SR, Wang F, Becker NA, Schule B, Kock N, Phillips LA, et al. Alterations in the common fragile site gene Parkin in ovarian and other cancers. Oncogene 2003; 22: 8370–8.
Glinka Y, Gassen M, Youdim MB . Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl 1997; 50: 55–66.
Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol 2001; 65: 135–72.
Li LY, Zhao XL, Fei XF, Gu ZL, Qin ZH, Liang ZQ . Bilobalide inhibits 6-OHDA-induced activation of NF-kappaB and loss of dopaminergic neurons in rat substantia nigra. Acta Pharmacol Sin 2008; 29: 539–47.
Park SY, Kim do Y, Kang JK, Park G, Choi YW . Involvement of activation of the Nrf2/ARE pathway in protection against 6-OHDA-induced SH-SY5Y cell death by alpha-iso-cubebenol. Neurotoxicology 2014; 44: 160–8.
Wang T, Liu YY, Wang X, Yang N, Zhu HB, Zuo PP . Protective effects of octacosanol on 6-hydroxydopamine-induced Parkinsonism in rats via regulation of ProNGF and NGF signaling. Acta Pharmacol Sin 2010; 31: 765–74.
Masoudi N, Ibanez-Cruceyra P, Offenburger SL, Holmes A, Gartner A . Tetraspanin (TSP-17) protects dopaminergic neurons against 6-OHDA-induced neurodegeneration in C. elegans. PLoS Genet 2014; 10: e1004767.
Kulich SM, Chu CT . Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J Neurochem 2001; 77: 1058–66.
Runden E, Seglen PO, Haug FM, Ottersen OP, Wieloch T, Shamloo M, et al. Regional selective neuronal degeneration after protein phosphatase inhibition in hippocampal slice cultures: evidence for a MAP kinase-dependent mechanism. J Neurosci 1998; 18: 7296–305.
Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem 2000; 275: 12200–6.
Zhu JH, Guo F, Shelburne J, Watkins S, Chu CT . Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol 2003; 13: 473–81.
Vercammen L, Van der Perren A, Vaudano E, Gijsbers R, Debyser Z, Van den Haute C, et al. Parkin protects against neurotoxicity in the 6-hydroxydopamine rat model for Parkinson's disease. Mol Ther 2006; 14: 716–23.
Hyun DH, Lee M, Halliwell B, Jenner P . Effect of overexpression of wild-type or mutant parkin on the cellular response induced by toxic insults. J Neurosci Res 2005; 82: 232–44.
Weissman AM . Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol 2001; 2: 169–78.
Ikeda F, Dikic I . Atypical ubiquitin chains: new molecular signals. 'Protein Modifications: Beyond the Usual Suspects' review series. EMBO Rep 2008; 9: 536–42.
Nathan JA, Kim HT, Ting L, Gygi SP, Goldberg AL . Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes? EMBO J 2013; 32: 552–65.
Moscat J, Diaz-Meco MT, Wooten MW . Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci 2007; 32: 95–100.
Acknowledgements
This work was supported in part by the National Natural Sciences Foundation of China (No 31330030 and 81371393), National Basic Research Program of China (973 Program) (2011CB504102), the Natural Science Foundation of Jiangsu Province (BK20140328), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hou, Xo., Si, Jm., Ren, Hg. et al. Parkin represses 6-hydroxydopamine-induced apoptosis via stabilizing scaffold protein p62 in PC12 cells. Acta Pharmacol Sin 36, 1300–1307 (2015). https://doi.org/10.1038/aps.2015.54
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2015.54
Keywords
This article is cited by
-
A small molecule antagonist of SMN disrupts the interaction between SMN and RNAP II
Nature Communications (2022)
-
UFBP1, a key component in ufmylation, enhances drug sensitivity by promoting proteasomal degradation of oxidative stress-response transcription factor Nrf2
Oncogene (2021)
-
Cereblon attenuates DNA damage-induced apoptosis by regulating the transcription-independent function of p53
Cell Death & Disease (2019)
-
Low Molecular Weight Sulfated Chitosan: Neuroprotective Effect on Rotenone-Induced In Vitro Parkinson’s Disease
Neurotoxicity Research (2019)
-
The Catalytically Inactive Mutation of the Ubiquitin-Conjugating Enzyme CDC34 Affects its Stability and Cell Proliferation
The Protein Journal (2018)