Abstract
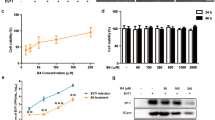
Human enterovirus 71 (EV71) is the primary causative agent of recent large-scale outbreaks of hand, foot, and mouth disease (HFMD) in Asia. Currently, there are no drugs available for the prevention and treatment of HFMD. In this study, we compared the anti-EV71 activities of three natural compounds, rheum emodin, artemisinin and astragaloside extracted from Chinese herbs Chinese rhubarb, Artemisia carvifolia and Astragalus, respectively, which have been traditionally used for the treatment and prevention of epidemic diseases. Human lung fibroblast cell line MRC5 was mock-infected or infected with EV71, and treated with drugs. The cytotoxicity of the drugs was detected with MTT assay. The cytopathic effects such as cell death and condensed nuclei were morphologically observed. The VP1-coding sequence required for EV71 genome replication was assayed with qRT-PCR. Viral protein expression was analyzed with Western blotting. Viral TCID50 was determined to evaluate EV71 virulence. Flow cytometry analysis of propidium iodide staining was performed to analyze the cell cycle distribution of MRC5 cells. Rheum emodin (29.6 μmol/L) effectively protected MRC5 cells from EV71-induced cytopathic effects, which resulted from the inhibiting viral replication: rheum emodin treatment decreased viral genomic levels by 5.34-fold, viral protein expression by less than 30-fold and EV71 virulence by 0.33107-fold. The fact that inhibition of rheum emodin on viral virulence was much stronger than its effects on genomic levels and viral protein expression suggested that rheum emodin inhibited viral maturation. Furthermore, rheum emodin treatment markedly diminished cell cycle arrest at S phase in MRC5 cells, which was induced by EV71 infection and favored the viral replication. In contrast, neither astragaloside (50 μmol/L) nor artemisinin (50 μmol/L) showed similar anti-EV71 activities. Among the three natural compounds tested, rheum emodin effectively suppressed EV71 viral replication, thus is a candidate anti-HFMD drug.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wang X, Zhu C, Bao W, Zhao K, Niu J, Yu XF, et al. Characterization of full-length enterovirus 71 strains from severe and mild disease patients in northeastern China. PLoS One 2012; 7: e32405.
Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE . Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis 2003; 9: 78–85.
Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J 2010; 7: 94.
Fan X, Jiang J, Liu Y, Huang X, Wang P, Liu L, et al. Detection of human enterovirus 71 and Coxsackievirus A16 in an outbreak of hand, foot, and mouth disease in Henan Province, China in 2009. Virus Genes 2012; 46: 1–9.
Liu MY, Liu W, Luo J, Liu Y, Zhu Y, Berman H, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One 2011; 6: e25287.
Li J, Huo X, Dai Y, Yang Z, Lei Y, Jiang Y, et al. Evidences for intertypic and intratypic recombinant events in EV71 of hand, foot and mouth disease during an epidemic in Hubei Province, China, 2011. Virus Res 2012; 169: 195–202.
Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, et al. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin Infect Dis 2000; 31: 678–83.
Shimizu H, Utama A, Yoshii K, Yoshida H, Yoneyama T, Sinniah M, et al. Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1997-1998. Jpn J Infect Dis 1999; 52: 12–5.
Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH . Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10: 778–90.
Yu JH, Zhang LY, Ren PY, Zhong T, Li ZL, Wang ZY, et al. Enterovirus 71 mediates cell cycle arrest in S phase through non-structural protein 3D. Cell Cycle 2015; 14: 425–36.
DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA . Privileged structures: applications in drug discovery. Comb Chem High Throughput Screen 2004; 7: 473–94.
Breinbauer R, Vetter IR, Waldmann H . From protein domains to drug candidates-natural products as guiding principles in the design and synthesis of compound libraries. Angew Chem Int Ed Engl 2002; 41: 2879–90.
Mann J . Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer 2002; 2: 143–8.
Si X, McManus BM, Zhang J, Yuan J, Cheung C, Esfandiarei M, et al. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J Virol 2005; 79: 8014–23.
Si X, Wang Y, Wong J, Zhang J, McManus BM, Luo H . Dysregulation of the ubiquitin-proteasome system by curcumin suppresses coxsackievirus B3 replication. J Virol 2007; 81: 3142–50.
Huang Q, Lu G, Shen HM, Chung MC, Ong CN . Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev 2007; 27: 609–30.
Schwarz S, Wang K, Yu W, Sun B, Schwarz W . Emodin inhibits current through SARS-associated coronavirus 3a protein. Antiviral Res 2011; 90: 64–9.
Shuangsuo D, Zhengguo Z, Yunru C, Xin Z, Baofeng W, Lichao Y, et al. Inhibition of the replication of hepatitis B virus in vitro by emodin. Med Sci Monit 2006; 12: BR302–6.
Xiong HR, Luo J, Hou W, Xiao H, Yang ZQ . The effect of emodin, an anthraquinone derivative extracted from the roots of Rheum tanguticum, against herpes simplex virus in vitro and in vivo. J Ethnopharmacol 2010; 133: 718–23.
Yeung S, Pongtavornpinyo W, Hastings IM, Mills AJ, White NJ . Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am J Trop Med Hyg 2004; 71: 179–86.
Chen P, Xie Y, Shen E, Li GG, Yu Y, Zhang CB, et al. Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-beta1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol 2011; 658: 168–74.
Gay RT, Belisle S, Beck MA, Meydani SN . An aged host promotes the evolution of avirulent coxsackievirus into a virulent strain. Proc Natl Acad Sci U S A 2006; 103: 13825–30.
Reed LJ . A simple method of estimating fifty percent endpoints. Am J Hyg 1983; 27: 493–7.
Shen FH, Shen TJ, Chang TM, Su IJ, Chen SH . Early dexamethasone treatment exacerbates enterovirus 71 infection in mice. Virology 2014; 464-465: 218–27.
Flobinus A, Taudon N, Desbordes M, Labrosse B, Simon F, Mazeron MC, et al. Stability and antiviral activity against human cytomegalovirus of artemisinin derivatives. J Antimicrob Chemother 2013; 69: 34–40.
Lin CJ, Lin HJ, Chen TH, Hsu YA, Liu CS, Hwang GY, et al. Polygonum cuspidatum and its active components inhibit replication of the influenza virus through toll-like receptor 9-induced interferon beta expression. PLoS One 2015; 10: e0117602.
Zhang Y, Zhu H, Huang C, Cui X, Gao Y, Huang Y, et al. Astragaloside IV exerts antiviral effects against coxsackievirus B3 by upregulating interferon-gamma. J Cardiovasc Pharmacol 2006; 47: 190–5.
Yu J, Zhang L, Ren P, Zhong T, Li Z, Wang Z, et al. Enterovirus 71 mediates cell cycle arrest in S phase through non-structural protein 3D. Cell Cycle 2015; 14: 425–36.
Jin P, Li J, Zhang X, Meng F, Zhou Y, Yao X, et al. Validation and evaluation of serological correlates of protection for inactivated enterovirus 71 vaccine in children aged 6–35 months. Hum Vaccin Immunother 2016; 12: 916–21.
Zhu FC, Liang ZL, Li XL, Ge HM, Meng FY, Mao QY, et al. Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 2013; 381: 1037–45.
Wang L, Wang J, Ma S, Liu Y . Anti-enterovirus 71 agents of natural products. Molecules 2015; 20: 16320–33.
Wu Q, Chen W, Sinha B, Tu Y, Manning S, Thomas N, et al. Neuroprotective agents for neonatal hypoxic-ischemic brain injury. Drug Discov Today 2015; 20: 1372–81.
Burr AR, Molkentin JD . Genetic evidence in the mouse solidifies the calcium hypothesis of myofiber death in muscular dystrophy. Cell Death Differ 2015; 22: 1402–12.
Chang SC, Lin JY, Lo LY, Li ML, Shih SR . Diverse apoptotic pathways in enterovirus 71-infected cells. J Neurovirol 2004; 10: 338–49.
Wang YF, Chou CT, Lei HY, Liu CC, Wang SM, Yan JJ, et al. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J Virol 2004; 78: 7916–24.
Zeng M, Zheng X, Wei R, Zhang N, Zhu K, Xu B, et al. The cytokine and chemokine profiles in patients with hand, foot and mouth disease of different severities in Shanghai, China, 2010. PLoS Negl Trop Dis 2013; 7: e2599.
Pang X, Liu J, Li Y, Zhao J, Zhang X . Emodin inhibits homocysteine-induced C-reactive protein generation in vascular smooth muscle cells by regulating PPARgamma expression and ROS-ERK1/2/p38 signal pathway. PLoS One 2015; 10: e0131295.
Yang XJ, Liu J, Ye L, Liao QJ, Wu JG, Gao JR, et al. HCV NS2 protein inhibits cell proliferation and induces cell cycle arrest in the S-phase in mammalian cells through down-regulation of cyclin A expression. Virus Res 2006; 121: 134–43.
Helt AM, Harris E . S-phase-dependent enhancement of dengue virus 2 replication in mosquito cells, but not in human cells. J Virol 2005; 79: 13218–30.
Tang QH, Zhang YM, Fan L, Tong G, He L, Dai C . Classic swine fever virus NS2 protein leads to the induction of cell cycle arrest at S-phase and endoplasmic reticulum stress. Virol J 2010; 7: 4.
Acknowledgements
This work was supported by funding from the National Natural Science Foundation of China (81301416), the Postdoctoral Science Foundation of China (2014M561302 and 2015T80299), the Norman Bethune Program of Jilin University (2015202), the Jilin Provincial Science and Technology Department (20140204004YY and 20160414025GH), and the Department of Human Resources and Social Security of Jilin Province (2016014), the National Natural Science Foundation of Henan Province (132300410228).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, T., Zhang, Ly., Wang, Zy. et al. Rheum emodin inhibits enterovirus 71 viral replication and affects the host cell cycle environment. Acta Pharmacol Sin 38, 392–401 (2017). https://doi.org/10.1038/aps.2016.110
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2016.110
Keywords
This article is cited by
-
Enterovirus E infections in goats with respiratory disease
BMC Veterinary Research (2025)
-
The Effect of Rheum turkestanicum Janisch in the Treatment of Bacterial Vaginosis Recurrence: A triple-Blinded Randomized Clinical Trial
Journal of Pharmaceutical Innovation (2025)
-
Construction of recombinant fluorescent LSDV for high-throughput screening of antiviral drugs
Veterinary Research (2024)
-
RNA m6A methylation regulators in sepsis
Molecular and Cellular Biochemistry (2024)
-
The Himalayan rhubarb (Rheum australe D. Don.): an endangered medicinal herb with immense ethnobotanical ‘use-value’
Vegetos (2024)