Abstract
Aim:
Glucagon-like peptide-1 (GLP-1) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors can not only lower blood glucose levels, but also alleviate cardiac remodeling after myocardial ischemia and hypertension. In the present study, we investigated the effects of a DPP-4 inhibitor (linagliptin) and a GLP-1 activator (liraglutide) on glucose- and angiotensin II (Ang II)-induced collagen formation and cytoskeleton reorganization in cardiac fibroblasts in vitro, and elucidated the related mechanisms.
Methods:
Cardiac fibroblasts were isolated from the hearts of 6-week-old C57BL/6 mice, and then exposed to different concentrations of glucose or Ang II for 24 h. The expression of fibrotic signals (fibronectin, collagen-1, -3 and -4), as well as ERK1/2 and NF-κB-p65 in the fibroblasts was examined using Western blotting assays. F-actin degradation was detected under inverted laser confocal microscope in fibroblasts stained with Rhodamine phalloidin.
Results:
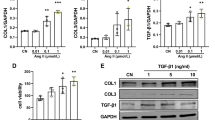
Glucose (1–40 mmol/L) and Ang II (10−8–10−5 mol/L) dose-dependently increased the expression of fibronectin, collagens, phospho-ERK1/2 and phospho-NF-κB-p65 in cardiac fibroblasts. High concentrations of glucose (≥40 mmol/L) and Ang II (≥10−6 mol/L) caused a significant degradation of F-actin (less assembly F-actin fibers and more disassembly fibers). ERK1/2 inhibitor U0126 (10 μmol/L) and NF-κB inhibitor JSH-23 (10 μmol/L) both markedly suppressed glucose- and angiotensin II-induced fibronectin and collagen expressions in cardiac fibroblasts. Furthermore, pretreatment with liraglutide (10–100 nmol/L) or linagliptin (3 and 30 nmol/L) significantly decreased glucose- and Ang II-induced expression of fibrotic signals, phospho-ERK1/2 and phospho-NF-κB-p65 in cardiac fibroblasts. Moreover, pretreatment with liraglutide (30 nmol/L) or liraglutide (100 nmol/L) markedly inhibited glucose-induced F-actin degradation, however, only liraglutide inhibited Ang II-induced F-actin degradation.
Conclusion:
Linagliptin and liraglutide inhibit glucose- and Ang II-induced collagen formation in cardiac fibroblasts via activation of the ERK/NF-κB/pathway. Linagliptin and liraglutide also markedly inhibit glucose-induced F-actin degradation in cardiac fibroblasts, but only liraglutide inhibits Ang II-induced F-actin degradation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lee TI, Kao YH, Chen YC, Huang JH, Hsu MI, Chen YJ . The dipeptidyl peptidase-4 inhibitor-sitagliptin modulates calcium dysregulation, inflammation, and PPARs in hypertensive cardiomyocytes. Int J Cardiol 2013; 168: 5390–5.
Zhao Y, Yang L, Zhou Z . Dipeptidyl peptidase-4 inhibitors: multitarget drugs, not only antidiabetes drugs. J Diabetes 2014; 6: 21–9.
Lenski M, Kazakov A, Marx N, Böhm M, Laufs U . Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J Mol Cell Cardiol 2011; 51: 906–18.
Picatoste B, Ramírez E, Caro-Vadillo A, Iborra C, Ares-Carrasco S, Egido J, et al. Sitagliptin reduces cardiac apoptosis, hypertrophy and fibrosis primarily by insulin-dependent mechanisms in experimental type-II diabetes. Potential roles of GLP-1 isoforms. PLoS One 2013; 8: e78330.
Connelly KA, Zhang Y, Advani A, Advani SL, Thai K, Yuen DA, et al. DPP-4 inhibition attenuates cardiac dysfunction and adverse remodeling following myocardial infarction in rats with experimental diabetes. Cardiovasc Ther 2013; 31: 259–67.
Johansen OE, Neubacher D, von Eynatten M, Patel S, Woerle HJ . Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-ananlysis of a phase 3 programme. Cardiovasc Diabetol 2012; 11: 3.
Nogueira KC, Furtado M, Fukui RT, Correia MR, Dos Santos RF, Andrade JL, et al. Left ventricular diastolic function in patients with type 2 diabetes treated with a dipeptidyl peptidase-4 inhibitor–a pilot study. Diabetol Metab Syndr 2014; 6: 103.
Hocher B, Sharkovska Y, Mark M, Klein T, Pfab T . The novel DPP-4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats. Int J Cardiol 2013; 167: 87–93.
Chinda K, Palee S, Surinkaew S, Phornphtkul M, Chattipakorn S, Chattipakorn N . Cardioprotective effect of dipeptidyl peptidae-4 inhibitor during ischemia-reperfusion injury. Int J Cardiol 2013; 167: 451–7.
Wang X, Lu J, Khaidakov M, Mitra S, Ding Z, Raina S, et al. Aspirin suppresses cardiac fibroblast proliferation and collagen formation through downregulation of angiotensin type 1 receptor transcription. Toxicol Appl Pharmacol 2012; 259: 346–54.
Wang X, Khaidakov M, Ding Z, Mercanti F, Mehta JL . LOX-1 in the maintenance of cytoskeleton and proliferation in senescent cardiac fibroblasts. J Mol Cell Cardiol 2013; 60: 184–90.
Tang M, Zhang W, Lin H, Jiang H, Dai H, Zhang Y . High glucose promotes the production of collagen types I and III by cardiac fibroblasts through a pathway dependent on extracellular-signal-regulated kinase 1/2. Mol Cell Biochem 2007; 301: 109–14.
Huang J, Siragy HM . Regulation of (pro) renin receptor expression by glucose-induced mitogen-activated protein kinase, nuclear factor-kappaB, and activator protein-1 signaling pathways. Endocrinology 2010; 151: 3317–25.
Madhyastha R, Madhyastha H, Pengam Y, Nakajima Y, Omura S, Maruyama M . NFkappaB activation is essential for miR-21 induction by TGFβ1 in high glucose conditions. Biochem Biophys Res Commun 2014; 451: 615–21.
Wang LP, Wang Y, Zhao LM, Li GR, Deng XL . Angiotensin II upregulates KCa3.1 channels and stimulates cell proliferation in rat cardiac fibroblasts. Biochem Pharmacol 2013; 85: 1486–94.
Choi C, Helfman DM . The Ras-ERK pathway modulates cytoskeleton organization, cell motility and lung metastasis signature genes in MDA-MB-231 LM2. Oncogene 2013; 33: 3669–76.
Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol 2007; 293: H3356–65.
Fiaschi T, Magherini F, Gamberi T, Lucchese G, Faggian G, Modesti A, et al. Hyperglycemia and angiotensin II cooperate to enhance collagen I deposition by cardiac fibroblasts through a ROS-STAT3-dependent mechanism. Biochim Biophys Acta 2014; 1843: 2603–10.
Copaja M, Venegas D, Aranguiz P, Canales J, Vivar R, Avalos Y, et al. Simvastatin disrupts cytoskeleton and decreases cardiac fibroblast adhesion, migration and viability. Toxicology 2012; 294: 42–9.
Schram K, Granguly R, No EK, Fang X, Thong FS, Sweeney G . Regulation of MT1-MMP and MMP-2 by leptin in cardiac fibroblasts involves Rho/ROCK-dependent actin cytoskeletal reorganization and leads to enhanced cell migration. Endocrinology 2011; 152: 2037–47.
Sassoli C, Chellini F, Pini A, Tani A, Nistri S, Nosi D, et al. Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-β/Smad3 signaling. PLoS One 2013; 8: e63896.
Lee TM, Lin SZ, Chang NC . Membrane ERα attenuates myocardial fibrosis via RhoA/ROCK-mediated actin remodeling in ovariectomized female infarcted rats. J Mol Med (Berl) 2014; 92: 43–51.
Shi D, Li X, Chen H, Che N, Zhou S, Lu Z, et al. High level of reactive oxygen species impaired mesenchymal stem cell migration via overpolymerization of F-actin cytoskeleton in systemic lupus erythematosus. Pathol Biol (Paris) 2014; 62: 382–90.
Surma M, Handy C, Chang J, Kapur R, Wei L, Shi J . ROCK1 deficiency enhances protective effects of antioxidants against apoptosis and cell detachment. PLoS One 2014; 9: e90758.
Kröller-Schön S, Knorr M, Hausding M, Oelze M, Schuff A, Schell R, et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res 2012; 96: 140–9.
Batchuluun B, Inoguchi T, Sonoda N, Sasaki S, Inoue T, Fujimura Y, et al. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis 2014; 232: 156–64.
Thakur S, Li L, Gupta S . NF-κB-mediated integrin-linked kinase regulation in angiotensin II-induced pro-fibrotic process in cardiac fibroblasts. Life Sci 2014; 107: 68–75.
Tang M, Zhong M, Shang Y, Lin H, Deng J, Jiang H, et al. Differential regulation of collagen type I and III expression in cardiac fibroblasts by AGEs through TRB3/MAPK signaling pathway. Cell Mol Life Sci 2008; 65: 2924–32.
Ferreira AJ, Shenoy V, Qi Y, Fraga-Silva RA, Santos RA, Katovich MJ, et al. Angiotensin-converting enzyme 2 activation protects against hypertension-induced cardiac fibrosis involving extracellular signal-regulated kinases. Exp Physiol 2011; 96: 287–94.
Ares-Carrasco S, Picatoste B, Benito-Martin A, Zubiri I, Sanz AB, Sánchez-Niño MD, et al. Myocardial fibrosis and apoptosis, but not inflammation, are present in long-term experimental diabetes. Am J Physiol Heart Circ Physiol 2009; 297: H2109–19.
Ogata T, Miyauchi T, Sakai S, Takanashi M, Irukayama-Tomobe Y, Yamaguchi I . Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J Am Coll Cardiol 2004; 43: 1481–8.
Villarreal F, Epperson SA, Ramirez-Sanchez I, Yamazaki KG, Brunton LL . Regulation of cardiac fibroblast collagen synthesis by adenosine: roles for Epac and PI3K. Am J Physiol Cell Physiol 2009; 296: C1178–84.
Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K . Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ 2006; 13: 730–7.
Acknowledgements
This study was supported by grants from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (Washington, DC, USA) (grant No BX-000282-05), the National Natural Science Foundation of China (grant No 81370428 and 31401246) and Boehringer Ingelheim Pharmaceuticals, Inc (Ridgefield, CT, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Xw., Zhang, Fx., Yang, F. et al. Effects of linagliptin and liraglutide on glucose- and angiotensin II-induced collagen formation and cytoskeleton degradation in cardiac fibroblasts in vitro. Acta Pharmacol Sin 37, 1349–1358 (2016). https://doi.org/10.1038/aps.2016.72
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2016.72
Keywords
This article is cited by
-
DPP-4 inhibition by linagliptin prevents cardiac dysfunction and inflammation by targeting the Nlrp3/ASC inflammasome
Basic Research in Cardiology (2019)
-
Anti-angiogenic effects of the DPP-4 inhibitor linagliptin via inhibition of VEGFR signalling in the mouse model of oxygen-induced retinopathy
Diabetologia (2018)
-
Dipeptidyl peptidase-4 (DPP-4) inhibition with linagliptin reduces western diet-induced myocardial TRAF3IP2 expression, inflammation and fibrosis in female mice
Cardiovascular Diabetology (2017)