Abstract
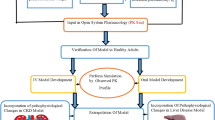
Caspofungin is an echinocandin antifungal agent licensed as a first-line therapy for invasive candidiasis in patients with moderate to severe illness or recent exposure to azoles. In this study we developed a whole-body physiology-based pharmacokinetics (WB-PBPK) model to predict the pharmacokinetics (PK) of caspofungin, and combined with Monte Carlo simulation (MCS) to optimize clinical dosage regimens of caspofungin in different kinds of patients. A WB-PBPK model of caspofungin was built and validated with raw data from 4 previous trials of general patients, intensive care unit (ICU) patients with Child-Pugh B, ICU patients on continuous renal replacement therapy, mild and moderate hepatic insuffciency (HI) patients. MCS was used to optimize clinical dosage regimens of caspofungin in these patients. A cumulative fraction of response (CFR) value of ≥90% was considered to be the minimum for achieving optimal empirical therapy. The simulated results of the WB-PBPK model were in good agreement with observed values of all trials. For general and ICU patients with caspofungin 70/50 mg, AUC and Cmax were decreased with the increase of body weight (BW) and showed great variation. MCS showed all general patients achieved CFR≥90% regardless of BW. But not all ICU patients with higher BW (≥70 kg) could achieve CFR≥90%. Compared with standard dosage regimens in general patients, caspofungin 70/35 mg in ICU patients with Child-Pugh B achieved significantly decreased AUC and Cmax, but obtained similar AUC and Cmax in moderate HI patients with Child-Pugh B. The WB-PBPK model of caspofungin is able to predict PK of all populations correctly. The combined WB-PBPK model with MCS can successfully optimize clinical dosage regimens of caspofungin in all patient populations.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48: 503–35.
Kofla G, Ruhnke M. Pharmacology and metabolism of anidulfungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature. Eur J Med Res 2011; 16: 159–66.
Walsh TJ, Adamson PC, Seibel NL, Flynn PM, Neely MN, Schwartz C, et al. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob Agents Chemother 2005; 49: 4536–45.
Stone JA, Holland SD, Wickersham PJ, Sterrett A, Schwartz M, Bonfiglio C, et al. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob Agents Chemother 2002; 46: 739–45.
Sandhu P, Lee W, Xu X, Leake BF, Yamazaki M, Stone JA, et al. Hepatic uptake of the novel antifungal agent caspofungin. Drug Metab Dispos 2005; 33: 676–82.
Balani SK, Xu X, Arison BH, Silva MV, Gries A, DeLuna FA, et al. Metabolites of caspofungin acetate, a potent antifungal agent, in human plasma and urine. Drug Metab Dispos 2000; 28: 1274–8.
Nguyen TH, Hoppe-Tichy T, Geiss HK, Rastall AC, Swoboda S, Schmidt J, et al. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J Antimicrob Chemother 2007; 60: 100–6.
Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. Introduction to drug pharmacokinetics in the critically ill patient. Chest 2012; 141: 1327–36.
Muilwijk EW, Schouten JA, Leeuwen HJ, Zanten AR, Lange DW, Colbers A, et al. Pharmacokinetics of caspofungin in ICU patients. J Antimicrob Chemother 2014; 69: 3294–9.
Mistry GC, Migoya E, Deutsch PJ, Winchell G, Hesney M, Li S, et al. Single- and multiple-dose administration of caspofungin in patients with hepatic insufficiency: implications for safety and dosing recommendations. J Clin Pharmacol 2007; 47: 951–61.
Muilwijk EW, Lempers VJ, Burger DM, Warris A, Pickkers P, Aarnoutse RE, et al. Impact of special patient populations on the pharmacokinetics of echinocandins. Expert Rev Anti Infect Ther 2015; 13: 799–815.
Wurthwein G, Young C, Lanvers-Kaminsky C, Hempel G, Trame MN, Schwerdtfeger R, et al. Population pharmacokinetics of liposomal amphotericin B and caspofungin in allogeneic hematopoietic stem cell recipients. Antimicrob Agents Chemother 2012; 56: 536–43.
Sinnollareddy MG, Roberts JA, Lipman J, Akova M, Bassetti M, De Waele JJ, et al. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: Data from multinational Defining Antibiotic Levels in Intensive care unit (DALI) patients Study. Crit Care 2015; 19: 33.
Rodriguez-Hernandez MJ, Ruiz-Perez de Pipaon M, Marquez-Solero M, Martin-Rico P, Caston-Osorio JJ, Guerrero-Sanchez FM, et al. [Candidemias: multicentre analysis in 16 hospitals in Andalusia (Spain)]. Enferm Infecc Microbiol Clin 2011; 29: 328–33.
Schmitt W, Willmann S. Physiology-based pharmacokinetic modeling: ready to be used. Drug Discov Today Technol 2004; 1: 449–56.
Wurthwein G, Cornely OA, Trame MN, Vehreschild JJ, Vehreschild MJGT, Farowski F, et al. Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis. Antimicrob Agents Chemother 2013; 57: 1664–71.
Weiler S, Seger C, Pfisterer H, Stienecke E, Stippler F, Welte R, et al. Pharmacokinetics of caspofungin in critically ill patients on continuous renal replacement therapy. Antimicrob Agents Chemother 2013; 57: 4053–7.
Jones HM, Dickins M, Youdim K, Gosset JR, Attkins NJ, Hay TL, et al. Application of PBPK modelling in drug discovery and development at Pfizer. Xenobiotica 2012; 42: 94–106.
Stader F, Wuerthwein G, Groll AH, Vehreschild JJ, Cornely OA, Hempel G. Physiology-based pharmacokinetics of caspofungin for adults and paediatrics. Pharm Res 2015; 32: 2029–37.
Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob Agents Chemother 2010; 54: 2497–506.
Jones HM, Parrott N, Jorga K, Lave T. A novel strategy for physiologically based predictions of human pharmacokinetics. Clin Pharmacokinet 2006; 45: 511–42.
Parrott N, Paquereau N, Coassolo P, Lave T. An evaluation of the utility of physiologically based models of pharmacokinetics in early drug discovery. J Pharm Sci 2005; 94: 2327–43.
Yang Q, Wang T, Xie J, Wang Y, Zheng X, Chen L, et al. Pharmacokinetic/ pharmacodynamic adequacy of echinocandins against Candida spp in intensive care unit patients and general patient populations. Int J Antimicrob Agents 2016; 47: 397–402.
Strougo A, Yassen A, Krauwinkel W, Danhof M, Freijer J. A semiphysiological population model for prediction of the pharmacokinetics of drugs under liver and renal disease conditions. Drug Metab Dispos 2011; 39: 1278–87.
Brill MJ, Diepstraten J, Rongen A, Kralingen S, Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet 2012; 51: 277–304.
Jain R, Chung SM, Jain L, Khurana M, Lau SW, Lee JE, et al. Implications of obesity for drug therapy: limitations and challenges. Clin Pharmacol Ther 2011; 90: 77–89.
Knibbe CA, Brill MJ, Rongen A, Diepstraten J, Graaf PH, Danhof M. Drug disposition in obesity: toward evidence-based dosing. Annu Rev Pharmacol Toxicol 2015; 55: 149–67.
Vorwerk CK, Tuchen S, Streit F, Binder L, Hofmuller W, Behrens-Baumann W. Aqueous humor concentrations of topically administered caspofungin in rabbits. Ophthalmic Res 2009; 41: 102–5.
Edginton AN, Theil FP, Schmitt W, Willmann S. Whole body physiologically-based pharmacokinetic models: their use in clinical drug development. Expert Opin Drug Metab Toxicol 2008; 4: 1143–52.
Acknowledgements
The authors gratefully thank Tangdu Hospital of Fourth Medical University for outstanding assistance in the software support (Gastroplus 9.0).
This work is supported by the National Natural Science Foundation of China (No 81473177 and 81672954) and the Shaanxi Provincial Natural Science Foundation (No 2016JM8015).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Information
Supplementary Figures
Rights and permissions
About this article
Cite this article
Yang, Qt., Zhai, Yj., Chen, L. et al. Whole-body physiology-based pharmacokinetics of caspofungin for general patients, intensive care unit patients and hepatic insufficiency patients. Acta Pharmacol Sin 39, 1533–1543 (2018). https://doi.org/10.1038/aps.2017.176
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.176
Keywords
This article is cited by
-
Administration and Dosing of Systemic Antifungal Agents in Pediatric Patients
Pediatric Drugs (2020)