Abstract
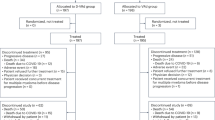
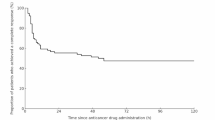
A double-blind, randomized, crossover study was conducted to compare the efficacy and safety of high-dose dexamethasone (Protocol D) with a combination of dexamethasone, metoclopramide and diphenhydramine (Protocol DMD) in the management of chemotherapy-induced nausea and vomiting in cancer patients. All entered patients had received no prior chemotherapy. During the study chemotherapy was administered on an inpatient basis. The majority of patients (94%) were treated with cytotoxic drugs of significant emetogenic activity and 40% of the study group received cis-platin-containing combinations. Of the 60 evaluable patients, complete antinausea and antivomiting effects of D were observed in 30 (50%) and 34 (57%), respectively and of DMD in 17 (28%) and 26 patients (43%) respectively. The difference was not statistically significant (P = 0.09 and 0.24, respectively). Lack of significant difference between the two regimens was demonstrated irrespective of the administered cytotoxic drugs. The DMD protocol caused more adverse reactions than D. While 27 patients (45%) experienced no side effects from D, only 14 (24%) remained free of complications due to DMD (P = 0.001). Furthermore, DMD produced more sedation, insomnia, headache, diaphoresis, dizziness and diarrhoea than the D regimen. In addition it gave rise to more adverse effects on appetite and activity. Upon direct questioning, 37 patients (62%) expressed a preference for D, 14 (23%) preferred DMD and 9 (15%) found no difference between the two regimens. We conclude that, while the short DMD protocol has an antiemetic activity equivalent in its effectiveness to D, its associated adverse reactions would minimize its usefulness. Therefore, further investigations should be conducted to find a safer and more potent combination of antiemetics suitable for therapy in an outpatient setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
al-Idrissi, H., Ibrahim, E., Abdullah, K. et al. Antiemetic efficacy of high-dose dexamethasone: Randomized, double-blind, crossover study with a combination of dexamethasone, metoclopramide and diphenhydramine. Br J Cancer 57, 308–312 (1988). https://doi.org/10.1038/bjc.1988.68
Issue date:
DOI: https://doi.org/10.1038/bjc.1988.68