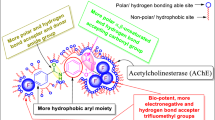
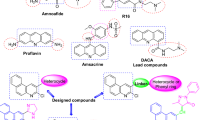
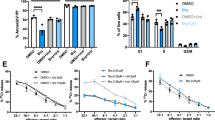
Abstract
As part of an 'enzyme-directed' approach to bioreductive drug development, we have measured the activity of NADH: cytochrome b5 reductase (B5R) in human cancer cell lines in order to assess the role of this enzyme in activating bioreductive drugs, and thus in influencing the cytotoxicity of these compounds. At present, there is no validated assay reported in the literature for measuring the activity of B5R in tumour cells, and current measurements have assumed that the enzyme activity can be measured either as the NADH-dependent reduction of cytochrome c or as the non-dicoumarol-inhibitable activity in the DT-diaphorase assay. Using p-hydroxymercuribenzoate (pHMB) as an inhibitor of B5R, we have quantified the contribution of B5R to the NADH-dependent reduction of cytochrome c and to the overall reduction of cytochrome c in the DT-diaphorase assay. In the former we found that residual uninhibited activity remained in the presence of pHMB, in some cases accounting for up to 60% of the total reduction of cytochrome c. Thus, simply measuring the NADH-dependent reduction of cytochrome c consistently overestimated B5R activity. We also found that the non-dicoumarol-inhibitable activity in the DT-diaphorase assay underestimated B5R activity, especially in cell lines with high DT-diaphorase activity. Therefore, we have developed a spectrophotometric assay for measuring B5R activity as the pHMB-inhibitable NADH-dependent reduction of cytochrome c. This has been used to measure the B5R activity of a panel of 22 human tumour cell lines, in which we found 7-fold and 3-fold variations in activity expressed per cell or per mg protein respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barham, H., Inglis, R., Chinje, E. et al. Development and validation of a spectrophotometric assay for measuring the activity of NADH: cytochrome b5 reductase in human tumour cells. Br J Cancer 74, 1188–1193 (1996). https://doi.org/10.1038/bjc.1996.515
Issue date:
DOI: https://doi.org/10.1038/bjc.1996.515
This article is cited by
-
Enhanced cytotoxicity of mitomycin C in human tumour cells with inducers of DT-diaphorase
British Journal of Cancer (1999)