Abstract
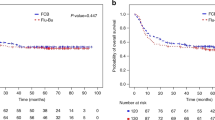
We performed a single institution retrospective analysis of 114 patients treated with BU-based pretransplant conditioning regimens. Oral BU was administered to 76 patients (total dose 16 mg/kg or 8 mg/kg) and i.v. BU to 38 others (total dose 12.8 mg/kg or 6.4 mg/kg). Either CY (n=74) or fludarabine (n=40) was given in combination with BU. Median age was 35 years in the oral BU group and 48.5 years with i.v. BU (P<0.001). OS and PFS rates at 3-years post HSCT were not different in patients who received either i.v. or oral BU (OS: 41.3 vs 44.0% (P=0.981); PFS: 52.7 vs 54.7% (P=0.526), respectively). The i.v. BU, however, was associated with a significantly shorter time to engraftment (13.5 days vs 16 days, respectively; P<0.001). There were no significant differences in survival or 100-day mortality for patients who received either CY or fludarabine, in combination with BU. After adjustment for confounders, multivariate analysis showed that age of transplant (P=0.002), donor type (sibling or unrelated; P=0.003), GVHD (P<0.05) and route of administration (P=0.023) were significant risk factors for OS. The i.v. BU used in an older age group yielded equivalent survival compared with oral BU used in a younger population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826.
Spurr C, Wilson W, Med J, McDonald J . Myleran in the treatment of chronic myeloid leukemia: results of treatment. South Med J 1956; 49: 847–855.
Sanders JE, Pritchard S, Mahoney P, Amos D, Buckner CD, Witherspoon RP et al. Growth and development following marrow transplantation for leukemia. Blood 1986; 68: 1129–1135.
McDonald G, Sharma P, Matthews D, Shulman H, Thomas E . The clinical course of 53 patients with venocclusive disease of the liver after marrow transplantation. Transplantation 1985; 36: 603–608.
Vassal G, Gouyette A, Hartmann O, Pico JL, Lemerle J . Pharmacokinetics of high-dose busulfan in children. Cancer Chemother Pharmacol 1989; 24: 386–390.
Krivoy N, Hoffer E, Lurie Y, Bentur Y, Rowe JM . Busulfan use in hematopoietic stem cell transplantation: pharmacology, dose adjustment, safety and efficacy in adults and children. Curr Drug Saf 2008; 3: 60–66.
Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS . Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol 1996; 37: 401–408.
Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant 2002; 8: 468–476.
Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med 1983; 309: 1347–1353.
Peters WP, Henner WD, Grochow LB, Olsen G, Edwards S, Stanbuck H et al. Clinical and pharmacologic effects of high dose single agent busulfan with autologous bone marrow support in the treatment of solid tumors. Cancer Res 1987; 47: 6402–6406.
Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood 2003; 102: 820–826.
Chunduri S, Dobogai LC, Peace D, Saunthararajah Y, Quigley J, Chen YH et al. Fludarabine/i.v. BU conditioning regimen: myeloablative, reduced intensity or both? Bone Marrow Transplant 2008; 41: 935–940.
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004; 104: 857–864.
Kröger N, Bornhäuser M, Ehninger G, Schwerdtfeger R, Biersack H, Sayer HG et al. Allogeneic stem cell transplantation after a fludarabine/busulfan-based reduced-intensity conditioning in patients with myelodysplastic syndrome or secondary acute myeloid leukemia. Ann Hematol 2003; 82: 336–342.
Nath CE, Shaw PJ . Busulphan in blood and marrow transplantation: dose, route, frequency and role of therapeutic drug monitoring. Curr Clin Pharmacol 2007; 2: 75–91.
Chae YS, Sohn SK, Kim JG, Cho YY, Moon JH, Shin HJ et al. New myeloablative conditioning regimen with fludarabine and busulfan for allogeneic stem cell transplantation: comparison with BuCy2. Bone Marrow Transplant 2007; 40: 541–547.
Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant 2002; 8: 493–500.
Lee JH, Choi SJ, Lee JH, Kim SE, Park CJ, Chi HS et al. Decreased incidence of hepatic veno-occlusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan + cyclophosphamide for allogeneic bone marrow transplantation. Ann Hematol 2005; 84: 321–330.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol 1991; 28: 250–259.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Bishop JF . The treatment of adult acute myeloid leukemia. Semin Oncol 1997; 24: 57–69.
Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer 2004; 101: 2788–2801.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783.
Slattery JT . Re: intravenous versus oral busulfan—perhaps not as different as suggested. Biol Blood Marrow Transplant 2003; 9: 282–284.
McCune JS, Holmberg LA . Busulfan in hematopoietic stem cell transplant setting. Expert Opin Drug Metab Toxicol 2009; 5: 957–969.
Toubai T, Paczesny S, Shono Y, Tanaka J, Lowler KP, Malter CT et al. Mesenchymal stem cells for treatment and prevention of graft-versus-host disease after allogeneic hematopoietic cell transplantation. Curr Stem Cell Res Ther 2009; 4: 252–259.
Koreth J, Aldridge J, Kim HT, Alyea EP, Cutler C, Armand P et al. Reduced-intensity conditioning hematopoietic stem cell transplantation in patients over 60 years: hematologic malignancy outcomes are not impaired in advanced age. Biol Blood Marrow Transplant 2010; 16: 792–800.
McCune JS, Gibbs JP, Slattery JT . Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet 2000; 39: 155–165.
Radich JP, Gooley T, Bensinger W, Chauncey T, Clift R, Flowers M et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood 2003; 102: 31–35.
Acknowledgements
We are thankful to Dr John Rush for his invaluable comments and editorial assistance with the manuscript. We also gratefully acknowledge Zhang Xue Hui and Lee Jing Jing for their help in collating the database. We would also like to thank the support of our hematology pharmacists, nurses and medical colleagues. This work was supported by grant support from the Singapore Cancer Syndicate and the National Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wong, A., Allen, J., Goh, Y. et al. Single center retrospective analysis of BU-based conditioning regimens in allogeneic transplantation. Bone Marrow Transplant 47, 181–189 (2012). https://doi.org/10.1038/bmt.2011.43
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/bmt.2011.43