Abstract
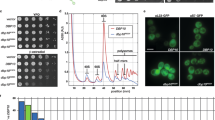
P68 RNA helicase is a prototypical DEAD box RNA helicase. The protein plays a very important role in early organ development and maturation. Consistent with the function of the protein in transcriptional regulation and pre-mRNA splicing, p68 was found to predominately localize in the cell nucleus. However, recent experiments demonstrate a transient cytoplasmic localization of the protein. We report here that p68 shuttles between the nucleus and the cytoplasm. The nucleocytoplasmic shuttling of p68 is mediated by two nuclear localization signal and two nuclear exporting signal sequence elements. Our experiments reveal that p68 shuttles via a classical RanGTPase-dependent pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Goldfarb DS, Gariepy J, Schoolnik G, et al. Synthetic peptides as nuclear localization signals. Nature 1986; 322:641–644.
Schneider J, Schindewolf C, van Zee K, et al. A mutant SV40 large T antigen interferes with nuclear localization of a heterologous protein. Cell 1988; 54:117–125.
Wen W, Meinkoth, JL, Tsien, RY, et al. Identification of a signal for rapid export of proteins from the nucleus. Cell 1995; 82:463–473.
Nakielny S, Dreyfuss G . Transport of proteins and RNAs in and out of the nucleus. Cell 1999; 99:677–690.
Gorlich D, Kutay U . Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 1999; 15:607–660.
Hill CS . Nucleocytoplasmic shuttling of Smad proteins. Cell Res 2009; 19:36–46.
Lusk CP, Blobel G, King MC . Highway to the inner nuclear membrane: rules for the road. Nat Rev Mol Cell Biol 2007; 8:414–420.
Crawford L, Leppard K, Lane D, et al. Cellular proteins reactive with monoclonal antibodies directed against simian virus 40 T-antigen. J Virol 1982; 42:612–620.
Lane DP, Hoeffler WK . SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature 1980; 288:167–170.
Iggo RD, Lane DP . Nuclear protein p68 is an RNA-dependent ATPase. EMBO J 1989; 8:1827–1831.
Ford MJ, Anton IA, Lane DP . Nuclear protein with sequence homology to translation initiation factor eIF-4A. Nature 1988; 332:736–738.
Hirling H, Scheffner M, Restle T, et al. RNA helicase activity associated with the human p68 protein. Nature 1989; 339:562–564.
Stevenson RJ, Hamilton SJ, MacCallum DE, et al. Expression of the 'dead box' RNA helicase p68 is developmentally and growth regulated and correlates with organ differentiation/maturation in the fetus. J Pathol 1998; 184:351–359.
Jost JP, Schwarz S, Hess D, et al. A chicken embryo protein related to the mammalian DEAD box protein p68 is tightly associated with the highly purified protein-RNA complex of 5- MeC-DNA glycosylase. Nucleic Acids Res 1999; 27: 3245–3252.
Liu ZR . p68 RNA helicase is an essential human splicing factor that acts at the U1 snRNA-5′ splice site duplex. Mol Cell Biol 2002; 22:5443–5450.
Lin C, Yang L, Yang JJ, et al. ATPase/helicase activities of p68 RNA helicase are required for pre-mRNA splicing but not for assembly of the spliceosome. Mol Cell Biol 2005; 25:7484–7493.
Fujita T, Kobayashi Y, Wada O, et al. Full activation of estrogen receptor alpha activation function-1 induces proliferation of breast cancer cells. J Biol Chem 2003; 278:26704–26714.
Watanabe M, Yanagisawa J, Kitagawa H, et al. A subfamily of RNA-binding DEAD-box proteins acts as an estrogen receptor alpha coactivator through the N-terminal activation domain (AF-1) with an RNA coactivator, SRA. EMBO J 2001; 20:1341–1352.
Rossow KL, Janknecht R . Synergism between p68 RNA helicase and the transcriptional coactivators CBP and p300. Oncogene 2003; 22:151–156.
Wilson BJ, Bates GJ, Nicol SM, et al. The p68 and p72 DEAD box RNA helicases interact with HDAC1 and repress transcription in a promoter-specific manner. BMC Mol Biol 2004; 5:11.
Endoh H, Maruyama K, Masuhiro Y, et al. Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. Mol Cell Biol 1999; 19:5363–5372.
Bates GJ, Nicol SM, Wilson BJ, et al. The DEAD box protein p68: a novel transcriptional coactivator of the p53 tumour suppressor. EMBO J 2005; 24:543–553.
Buszczak M, Spradling AC . The Drosophila P68 RNA helicase regulates transcriptional deactivation by promoting RNA release from chromatin. Genes Dev 2006; 20:977–989.
Kahlina K, Goren I, Pfeilschifter J, et al. p68 dead box RNA helicase expression in keratinocytes: regulation, nucleolar localization, and functional connection to proliferation and VEGF gene expression. J Biol Chem 2004; 279:44872–44882.
Yang L, Lin C, Liu ZR . Phosphorylations of DEAD box p68 RNA helicase are associated with cancer development and cell proliferation. Mol Cancer Res 2005; 3:355–363.
Wei Y, Hu MH . [The study of P68 RNA helicase on cell transformation]. Yi Chuan Xue Bao 2001; 28:991–996.
Warner DR, Bhattacherjee V, Yin X, et al. Functional interaction between Smad, CREB binding protein, and p68 RNA helicase. Biochem Biophys Res Commun 2004; 324:70–76.
Yang L, Liu ZR . Bacterially expressed recombinant p68 RNA helicase is phosphorylated on serine, threonine, and tyrosine residues. Protein Expr Purif 2004; 35:327–333.
Yang L, Lin C, Liu ZR . Signaling to the DEAD box-Regulation of DEAD-box p68 RNA helicase by protein phosphorylations. Cell Signal 2005; 17:1495–1504.
Yang L, Lin C, Liu ZR . P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing axin from beta-catenin. Cell 2006; 127:139–155.
Cartwright P, Helin K . Nucleocytoplasmic shuttling of transcription factors. Cell Mol Life Sci 2000; 57:1193–206.
Zhu J and McKeon F . Nucleocytoplasmic shuttling and the control of NF-AT signaling. Cell Mol Life Sci 2000; 57:411–420.
Fan XC, Steitz JA . Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 1998; 17:3448–3460.
Shibuya T, Tange TO, Sonenberg N, et al. eIF4AIII binds spliced mRNA in the exon junction complex and is essential for nonsense-mediated decay. Nat Struct Mol Biol 2004; 11:346–351.
Kalderon D, Roberts BL, Richardson WD, et al. A short amino acid sequence able to specify nuclear location. Cell 1984; 39 (Part 2):499–509.
Robbins J, Dilworth SM, Laskey RA, et al. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 1991; 64:615–623.
Neumann G, Hughes MT, Kawaoka Y . Influenza A virus NS2 protein mediates vRNP nuclear export through NES-independent interaction with hCRM1. EMBO J 2000; 19:6751–6758.
Fornerod M, Ohno M, Yoshida M, et al. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 1997; 90:1051–1060.
Watanabe M, Fukuda M, Yoshida M, et al. Involvement of CRM1, a nuclear export receptor, in mRNA export in mammalian cells and fission yeast. Genes Cells 1999; 4:291–297.
Goldfarb DS, Corbett AH, Mason DA, et al. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol 2004; 14:505–514.
Lindsay ME, Plafker K, Smith AE, et al. Npap60/Nup50 is a tri-stable switch that stimulates importin-alpha:beta-mediated nuclear protein import. Cell 2002; 110:349–360.
Nicol SM, Causevic M, Prescott AR, et al. The nuclear DEAD box RNA helicase p68 interacts with the nucleolar protein fibrillarin and colocalizes specifically in nascent nucleoli during telophase. Exp Cell Res 2000; 257:272–280.
Sheng Y, Tsai-Morris CH, Gutti R, et al. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is a transport protein involved in gene-specific mRNA export and protein translation during spermatogenesis. J Biol Chem 2006; 281:35048–35056.
Aratani S, Oishi T, Fujita H, et al. The nuclear import of RNA helicase A is mediated by importin-alpha3. Biochem Biophys Res Commun 2006; 340:125–133.
Askjaer P, Bachi A, Wilm M, et al. RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase. Mol Cell Biol 1999; 19:6276–6285.
Xu L, Massague J . Nucleocytoplasmic shuttling of signal transducers. Nat Rev Mol Cell Biol 2004; 5:209–219.
Kau TR, Way JC, Silver PA . Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer 2004; 4:106–117.
Loyola A, Almouzni G . Histone chaperones, a supporting role in the limelight. Biochim Biophys Acta 2004; 1677:3–11.
Fried H, Kutay U . Nucleocytoplasmic transport: taking an inventory. Cell Mol Life Sci 2003; 60:1659–1688.
Yang L, Lin C, Zhao S, et al. Phosphorylation of p68 RNA helicase plays a role in platelet-derived growth factor-induced cell proliferation by up-regulating cyclin D1 and c-Myc expression. J Biol Chem 2007; 282:16811–16819.
Davis BN, Hilyard AC, Lagna G, et al. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 2008; 454:56–61.
Fukuda T, Yamagata K, Fujiyama S, et al. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat Cell Biol 2007; 9:604–611.
Goh PY, Tan YJ, Lim SP, et al. Cellular RNA helicase p68 relocalization and interaction with the hepatitis C virus (HCV) NS5B protein and the potential role of p68 in HCV RNA replication. J Virol 2004; 78:5288–5298.
Harris D, Zhang Z, Chaubey B, et al. Identification of cellular factors associated with the 3'-nontranslated region of the hepatitis C virus genome. Mol Cell Proteomics 2006; 5:1006–1018.
Wrighton KH, Lin X, Feng XH . Phospho-control of TGF-beta superfamily signaling. Cell Res 2009; 19:8–20.
Niu Y, Roy F, Saltel F, et al. A nuclear export signal and phosphorylation regulate Dok1 subcellular localization and functions. Mol Cell Biol 2006; 26:4288–4301.
Okamura H, Aramburu J, Garcia-Rodriguez C, et al. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell 2000; 6:539–550.
Huang Y, Liu ZR . The ATPase, RNA unwinding, and RNA binding activities of recombinant p68 RNA helicase. J Biol Chem 2002; 277:12810–12815.
Wang H, Liu Y, Gao X, et al. The recombinant beta subunit of C-phycocyanin inhibits cell proliferation and induces apoptosis. Cancer Lett 2007; 247:150–158.
Cuff JA, Clamp ME, Siddiqui AS, et al. JPred: a consensus secondary structure prediction server. Bioinformatics 1998; 14:892–893.
McGuffin LJ, Bryson K, Jones DT . The PSIPRED protein structure prediction server. Bioinformatics 2000; 16:404–405.
Sengoku T, Nureki O, Nakamura A, et al. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell 2006; 125:287–300.
Acknowledgements
We thank Drs Joan A Steitz (Yale University School of Medicine), Melissa J Moore (University of Massachusetts medical school) and Hung-Ying Kao (Case Western Reserve University) for providing the vectors for expression of MS2-DEK and human CRM1. We are grateful to Professor Peter Stockley (University of Leeds) for providing antibody against MS2-DEK. We also thank Birgit Neuhaus (Georgia State University) for assistance in confocal imaging. This manuscript is greatly improved by comments from Christie Carter, Michael Kirberger and Heena Dey (Georgia State University). This work is supported in part by research grants from National Institutes of Health (GM063874 and CA118113) and Georgia Cancer Coalition to ZR Liu. X Gao is supported by an MBD fellowship, GSU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Gao, X., Huang, Y. et al. P68 RNA helicase is a nucleocytoplasmic shuttling protein. Cell Res 19, 1388–1400 (2009). https://doi.org/10.1038/cr.2009.113
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2009.113
Keywords
This article is cited by
-
Pisum sativum p68 DEAD-box protein is ATP-dependent RNA helicase and unique bipolar DNA helicase
Plant Molecular Biology (2014)