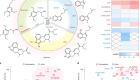
Researchers at Neuron-D want to accelerate the discovery of drugs for conditions such as Alzheimer's disease.Credit: Neuron-D
Neuron-D in Dresden, Germany, spun off from the German Center for Neurodegenerative Diseases in Göttingen and the Leibniz Institute for Polymer Research Dresden in 2019.
More than 95% of drug candidates for neurological diseases fail to come to market. One reason is that early-phase drug-testing systems use 2D cell cultures that don’t reflect the complexity of the human brain and its diseases.
Neuron-D, a start-up in Dresden, Germany, wants to change this with a hydrogel-based 3D cell-culture model in which neurons behave more as they do in people.
Read more about The Spinoff Prize
The company, formed in 2019 by scientists from the German Center for Neurodegenerative Diseases in Göttingen and the Leibniz Institute for Polymer Research Dresden, has been longlisted for The Spinoff Prize 2023. The company hopes that its technology will accelerate the discovery of drugs for brain cancer and Alzheimer’s disease, as well as help physicians to choose the most suitable drugs for individuals.
The human brain’s 86 billion neurons and nearly as many glial cells interact in a gel-like medium known as the extracellular matrix, which is intersected by some 650 kilometres of blood-carrying capillaries. A 2D culture of neurons and glial cells sitting side by side in a petri dish captures little of this.
Brain organoids — balls of tissue that self-organize from stem cells — can replicate neuronal connections, cell types and tissue organization. But no two organoids are exactly the same, which means each can respond differently to a drug. Simpler 3D cell models grown on animal-tissue scaffolds also vary from batch to batch.
By creating a synthetic hydrogel-based scaffold from scratch, with physical and chemical properties that can be precisely controlled, Neuron-D’s scientists have built what they say is a reproducible cell-culture system for high-throughput drug screening. Because organoids are thick balls of tissue, light cannot penetrate them easily. And most animal-tissue scaffolds are opaque, making imaging difficult. But Neuron-D’s hydrogel is transparent, so cell cultures can be imaged to record results in real time1.
Heparin, a sugar that binds to proteins, in the gel helps to control how growth factors are delivered to the cells. And cleavage peptides allow cells to attach to the gel and produce an extracellular matrix that behaves like the one in the brain.
Growing a network
A 20-microlitre hydrogel sphere with 10,000 neural progenitors can grow a functioning network of around 150,000 connected human neurons in less 3 weeks, says Neuron-D co-founder and scientific adviser Caghan Kizil, a neurobiologist now at Columbia University in New York City.
Part of Nature Outlook: The Spinoff Prize 2023
By tuning the properties of the gel to suit cell types, Neuron-D co-founders and advisers Uwe Freudenberg and Carsten Werner, both material scientists at the Leibniz Institute of Polymer Research Dresden, successfully cultured bone, cartilage, epithelium and blood-vessel cells.
Neuron-D’s first drug-discovery model is for glioblastoma multiforme — the cause of 60% of adult brain tumours and that has a median survival of around 14 months. A version for Alzheimer’s is in the pipeline2.
The technology can also be used to personalize treatment. The company can grow an individual’s glioblastoma cells, test a panel of drugs on them and find out which drugs work best, all within a fortnight. Neuron-D calls this personalized-medicine model its patient avatar. It plans to launch a glioblastoma multiforme version in 2024. That will be followed by an Alzheimer’s avatar, with the expectation that there will be a surge of approved disease-modifying Alzheimer’s drugs in the next few years.
Neuron-D plans to grow more cell types together to increase the predictive power of its system. This includes immune cells, which are an important aspect of many neurological diseases. “Every cell type brings a different pathological mechanism that can be investigated and drugged,” says Kizil.
“Neuron-D’s system has great potential,” says neurobiologist András Lakatos at the University of Cambridge, UK, who develops human organoids to model neurodegenerative diseases and is not involved with the company. But because it doesn’t represent all cell types — such as microglia or blood vessels — it has its limits. Ultimately, Lakatos says, the key to Neuron-D’s success will lie in how replicable the types of neuronal connection are, and whether disease hallmarks uniformly appear in specimens.

 The Spinoff Prize 2023
The Spinoff Prize 2023
 The Spinoff Prize: where are they now?
The Spinoff Prize: where are they now?
 Alpine Quantum: Commercializing quantum computers step by step
Alpine Quantum: Commercializing quantum computers step by step
 Jupiter Ionics: A cleaner route to ammonia
Jupiter Ionics: A cleaner route to ammonia
 Parity Quantum: Rewriting the quantum-computer blueprint
Parity Quantum: Rewriting the quantum-computer blueprint
 Resolve Stroke: Revealing vascular roadblocks in the brain
Resolve Stroke: Revealing vascular roadblocks in the brain
 SanaHeal: Bioglue breakthrough
SanaHeal: Bioglue breakthrough
 AquaLith: Better batteries built using existing technology
AquaLith: Better batteries built using existing technology
 Aural Analytics: Listening for neurological symptoms
Aural Analytics: Listening for neurological symptoms
 New Iridium: How to lower carbon levels using light
New Iridium: How to lower carbon levels using light
 ONA Therapeutics: A fat-blocking drug could help to fight metastatic cancer
ONA Therapeutics: A fat-blocking drug could help to fight metastatic cancer
 Senisca: RNA splicing targets age-related diseases
Senisca: RNA splicing targets age-related diseases
 Sibylla: Disrupting protein folding to tackle cancer
Sibylla: Disrupting protein folding to tackle cancer