Despite decades of innovation, the early stages of drug discovery remain a major bottleneck for pharmaceutical research and development (R&D). Traditional screening-based approaches rely heavily on existing chemical libraries and well-characterized scaffolds, aiming to optimize what is already known instead of discovering project-specific solutions. The result is an expensive, slow and uncertain process, with only half of discovery programs ever reaching preclinical development, and the cost of getting there doubling every nine years1.
Variational AI, a Vancouver-based generative artificial intelligence (AI) drug discovery startup, is solving this problem by shifting from screening to generating. At the heart of its technology is Enki, a generative AI foundation model specifically designed to discover and optimize novel small molecules. Unlike traditional screening methods, Enki navigates the chemical space beyond what is already known, proposing new chemical structures that are guided by the biological and pharmacological properties required for therapeutic success.
A new drug discovery paradigm
Enki is purpose-built for small-molecule drug discovery (Fig. 1). Fully trained on millions of data points including curated molecular structures, bioactivity profiles and properties across more than 700 drug targets, Enki has learned what defines a good drug molecule. It discovers structures unlikely to emerge from traditional experimental methods, and can generate diverse first-in-class and best-in-class structures that are both novel and synthesizable.
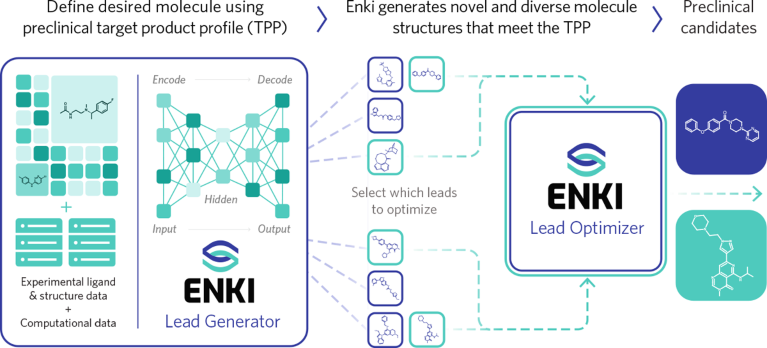
Fig. 1 | Enki’s drug discovery workflow.
First-generation discriminative AI algorithms used in drug discovery ranked large libraries of virtual molecules and then incrementally improved and refined hits into leads. Instead of taking that approach, Enki enables the navigation of previously unreachable regions of the chemical space with speed and precision, generating new lead-like compounds tailored to meet a particular target product profile (TPP) in weeks—or increasingly in just days. The molecules are inherently optimized across more than 50 properties, including potency, selectivity, toxicity, pharmacokinetics (PK)/absorption, distribution, metabolism and excretion (ADME), and synthetic tractability, enabling progress with fewer iteration cycles.
“Rather than screening and testing more and more compounds, Enki generates better molecules from the start—a radical change for small-molecule drug discovery,” explained Peter Guzzo, executive VP and head of drug discovery at Variational AI. “Whether targets are well-characterized or relatively unknown, our platform requires only a well-defined preclinical TPP as input, from which it proposes lead structures optimized for potency, selectivity, pharmacokinetics, toxicity and synthetic feasibility.”
In addition to rapidly generating novel and diverse lead compounds, Enki can perform multi-parametric lead optimization, rapidly enhancing compound profiles across multiple objectives while adhering to a fixed scaffold and specific constraints. By applying active learning, Enki helps research teams achieve greater progress with each iteration, reducing the number of design-make-test-analyze cycles required to reach a preclinical candidate.
New molecules, new possibilities
Using Enki, Variational AI has demonstrated success in real-world drug discovery projects, and is actively partnering with global pharmaceutical and biotechnology companies across oncology, dermatology, rare diseases and other high-need therapeutic areas. In collaborative engagements, Enki delivered potent leads with a high success rate, dramatically reducing the time and cost required to reach preclinical candidates. This performance has been replicated within industry, for example in accelerating the discovery of brain-penetrant ataxia telangiectasia and Rad3-related protein (ATR) inhibitors for oncology applications for Rakovina Therapeutics (Vancouver). Collaborations with Merck (known as MSD outside of the USA and Canada), ImmVue Therapeutics and other undisclosed biopharma companies have shown similar results, with Enki consistently generating high-quality sub-μM leads before any lead-optimization efforts.
“By using Enki to overcome the limitations of traditional screening or structure-based design, drug developers can rapidly explore the uncharted chemical space to generate high-quality and synthesizable lead compounds,” said Handol Kim, CEO of Variational AI. “This considerably de-risks and accelerates early programs.”
With a new Enki version released every quarter, Variational AI continues to offer partners state-of-the-art and rapidly evolving capabilities in generative drug design. “Whether companies are initiating a new program with complex targets or facing challenges in lead optimization, Enki can help them access innovative chemistry beyond the boundaries of conventional methods,” added Kim. “Enki is redefining the unit economics of drug discovery to bring much needed novel therapeutics to patients faster.”


