Abstract
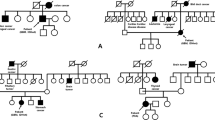
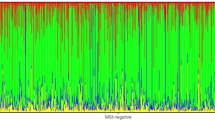
Microsatellite instability (MSI) is present in hereditary conditions due to mismatch repair (MMR) gene mutations. Following MSI analysis, tumor samples are classified into MSS (stable), MSI-L (low instability), and MSI-H (high instability) based on the fraction of unstable loci. Another MSI-based classification takes into account the size difference between mutant alleles in tumor DNA compared to wild-type alleles; two types of MSI, A and B, are recognized using this approach, type A being characterized by smaller, more subtle allelic shifts compared to type B. Biallelic mutations of MMR genes are associated with pediatric cancers, including glial tumors, in Turcot syndrome type 1 (TS1). However, most TS1-associated gliomas so far analyzed did not display MSI. We investigated the frequency of MSI in a series of 34 pediatric gliomas of different grade using a panel of five mononucleotide quasimonomorphic markers. Subtle qualitative changes were observed for the majority of markers in two glioblastomas (5.9% of the total series and 33.3% of glioblastomas). In both cases, family histories were compatible with TS1, and mutations of the PMS2 and MLH1 genes were identified. In one family, the MSI patterns were compared between the glioblastoma and a colon cancer from an affected relative, showing a clear qualitative difference, with the former displaying type A and the latter type B instability, respectively. These results were confirmed using additional microsatellite markers, indicating that knowledge of the association between TS1-related glial tumors and subtle type A MSI is important for full ascertainment of TS1 patients and appropriate counselling.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Turcot J, Despres JP, ST Pierre F : Malignant tumors of the central nervous system associated with familial polyposis of the colon: report of two cases. Dis Colon Rectum 1959; 2: 465–468.
Lucci-Cordisco E, Zito I, Gensini F, Genuardi M : Hereditary nonpolyposis colorectal cancer and related conditions. Am J Med Genet 2003; 122: 325–334.
Felton KEA, Gilchrist DM, Andrew SE : Constitutive deficiency in DNA mismatch repair. Clin Genet 2007; 71: 483–498.
Hamilton SR, Liu B, Parsons RE et al: The molecular basis of Turcot's syndrome. N Engl J Med 1995; 332: 839–847.
Paraf F, Jothy S, van Meir EG : Brain tumor-polyposis syndrome: two genetic diseases? J Clin Oncol 1997; 15: 2744–2758.
Boland CR, Thibodeau SN, Hamilton SR et al: A National Cancer Institute Workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998; 58: 5248–5257.
Thibodeau SN, Bren G, Schaid D : Microsatellite instability in cancer of the proximal colon. Science 1993; 260: 816–819.
Oda S, Mashara Y, Ikeda Y et al: Two modes of microsatellite instability in human cancer: differential connection of defective DNA mismatch repair to dinucleotide repeat instability. Nucleic Acids Res 2005; 33: 1628–1636.
Alonso M, Hamelin R, Kim M et al: Microsatellite instability occurs in distinct subtypes of pediatric but not adult central nervous system tumors. Cancer Res 2001; 61: 2124–2128.
Leung SY, Chan TL, Chung LP et al: Microsatellite instability and mutation of DNA mismatch repair genes in gliomas. Am J Pathol 1998; 153: 1181–1188.
Cheng Y, Ng HK, Zhang SF et al: Genetic alterations in pediatric high-grade astrocytomas. Hum Pathol 1999; 30: 1284–1290.
Kanamori M, Kon H, Nobukuni T et al: Microsatellite instability and the PTEN1 gene mutation in a subset of early onset gliomas carrying germline mutation or promoter methylation of the hMLH1 gene. Oncogene 2000; 19: 1564–1571.
Szybka M, Bartkowiak J, Zakrzewski K, Polis L, Liberski P, Kordek R : Microsatellite instability and expression of DNA mismatch repair genes in malignant astrocytic tumors from adult and pediatric patients. Clin Neuropathol 2003; 22: 180–186.
Eckert A, Kloor M, Giersch A et al: Microsatellite instability in pediatric and adult high-grade gliomas. Brain Pathol 2007; 17: 146–150.
Vladimirova V, Denkhaus D, Soerensen N, Wagner S, Wolff JE, Pietsch T : Low level microsatellite instability in paediatric malignant astrocytomas. Neuropathol Appl Neurobiol 2007; 34: 547–554.
Bougeard G, Charbonnier F, Moerman A et al: Early onset brain tumor and lymphoma in MSH2-deficient children. Am J Hum Genet 2003; 72: 213–216.
Menko FH, Kaspers GL, Meijer GA, Claes K, van Hagen JM, Gille JJ : A homozygous MSH6 mutation in a child with cafe-au-lait spots, oligodendroglioma and rectal cancer. Fam Cancer 2004; 3: 123–127.
Agostini M, Tibiletti MG, Lucci-Cordisco E et al: Two PMS2 mutations in a Turcot syndrome family with small bowel cancers. Am J Gastroenterol 2005; 100: 1886–1891.
Hegde MR, Chong B, Blazo ME et al: A homozygous mutation in MSH6 causes Turcot syndrome. Clin Cancer Res 2005; 11: 4689–4693.
de la Chapelle A : Testing tumors for microsatellite instability. Eur J Hum Genet 1999; 7: 407–408.
Buhard O, Cattaneo F, Wong YF et al: Multipopulation analysis of polymorphisms in five mononucleotide repeats used to determine the microsatellite instability status of human tumors. J Clin Oncol 2006; 24: 241–251.
Pastrello C, Baglioni S, Tibiletti MG et al: Stability of BAT26 in tumours of hereditary nonpolyposis colorectal cancer patients with MSH2 intragenic deletion. Eur J Hum Genet 2006; 14: 63–68.
Suraweera N, Duval A, Reperant M et al: Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology 2002; 123: 1804–1811.
Louis DN, Ohgaki H, Wiestler OD et al: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109.
Coombs NJ, Gough AC, Primrose JN : Optimisation of DNA and RNA extraction from archival formalin-fixed tissue. Nucleic Acids Res 1999; 27: e12.
Clendenning M, Hampel H, LaJeunesse J et al: Long-range PCR facilitates the identification of PMS2-specific mutations. Hum Mutat 2006; 27: 490–495.
Wooster R, Cleton-Jansen AM, Collins N et al: Instability of short tandem repeats microsatellites in human cancers. Nat Genet 1994; 6: 152–156.
Mizoguchi M, Inamura T, Ikezaki K et al: Patient survival and microsatellite instability in gliomas by high-resolution fluorescent analysis. Oncol Rep 1999; 6: 791–795.
Martinez R, Schackert HK, Plaschke J, Baretton G, Appelt H, Schackert G : Molecular mechanisms associated with chromosomal and microsatellite instability in sporadic glioblastoma multiforme. Oncology 2004; 66: 395–403.
Wagner A, Reddinguis R, Kros J et al: Wilms tumor and glioblastoma in a child with a double MLH1 germline mutation. Fam Cancer 2003; 1 (suppl.): 57.
Poley J-W, Wagner A, Hoogmans MCP et al: Biallelic germline mutations of mismatch-repair genes. Cancer 2007; 109: 2349–2356.
Duval A, Reperant M, Compoint A et al: Target gene mutation profile differs between gastrointestinal and endometrial tumors with mismatch repair deficiency. Cancer Res 2002; 62: 1609–1612.
Nakagawa H, Lockman JC, Frankel WL et al: Mismatch repair gene PMS2: disease-causing germline mutations are frequent in patients whose tumors stain negative for PMS2 protein, but paralogous genes obscure mutation detection and interpretation. Cancer Res 2004; 64: 4721–4727.
Gottschling S, Reinhard H, Pagenstecher C et al: Hypothesis: possible role of retinoic acid therapy in patients with biallelic mismatch repair gene defects. Eur J Pediatr 2008; 167: 225–229.
Scott RH, Homfray T, Huxter NL et al: Familial T-cell non-Hodgkin lymphoma caused by biallelic MSH2 mutations. J Med Genet 2007; 44: e83.
Jackson CC, Holter S, Pollett A et al: Cafè-au-lait macules and pediatric malignancy caused by biallelic mutations in the DNA mismatch repair (MMR) gene PMS2. Pediatr Blood Cancer 2008; 50: 1268–1270.
Tan TY, Orme LM, Lynch E et al: Biallelic PMS2 mutations and a distinctive childhood cancer syndrome. J Pediatr Hematol Oncol 2008; 30: 254–257.
Acknowledgements
This work was supported by a PRIN grant of the Italian Ministry for University to MG and by contributions of Ente Cassa di Risparmio di Firenze to Fiorgen and MG. We are grateful to Roberta Sestini and Benedetta Toschi for assistance with MLPA analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giunti, L., Cetica, V., Ricci, U. et al. Type A microsatellite instability in pediatric gliomas as an indicator of Turcot syndrome. Eur J Hum Genet 17, 919–927 (2009). https://doi.org/10.1038/ejhg.2008.271
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2008.271
Keywords
This article is cited by
-
Sporadic and Lynch syndrome-associated mismatch repair-deficient brain tumors
Laboratory Investigation (2022)
-
Immunohistochemical screening for mismatch repair protein deficiency in paediatric high-grade gliomas — institutional experience and review of literature
Child's Nervous System (2021)
-
Chemotherapy induced microsatellite instability and loss of heterozygosity in chromosomes 2, 5, 10, and 17 in solid tumor patients
Cancer Cell International (2014)
-
Evaluation of a new panel of six mononucleotide repeat markers for the detection of DNA mismatch repair-deficient tumours
British Journal of Cancer (2013)