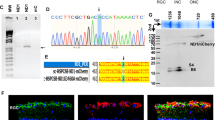
Abstract
Primary mitochondrial disorders occur at a prevalence of one in 10 000; ∼50% of these demonstrate ocular pathology. Leber hereditary optic neuropathy (LHON) is the most common primary mitochondrial disorder. LHON results from retinal ganglion cell pathology, which leads to optic nerve degeneration and blindness. Over 95% of cases result from one of the three common mutations in mitochondrial genes MTND1, MTND4 and MTND6, which encode elements of the complex I respiratory chain. Various therapies for LHON are in development, for example, intravitreal injection of adeno-associated virus carrying either the yeast NDI1 gene or a specific subunit of mammalian Complex I have shown visual improvement in animal models. Given the course of LHON, it is likely that in many cases prompt administration may be necessary before widespread cell death. An alternative approach for therapy may be the use of stem cells to protect visual function; this has been evaluated by us in a rotenone-induced model of LHON. Freshly dissected embryonic retinal cells do not integrate into the ganglion cell layer (GCL), unlike similarly obtained photoreceptor precursors. However, cultured retinal progenitor cells can integrate in close proximity to the GCL, and act to preserve retinal function as assessed by manganese-enhanced magnetic resonance imaging, optokinetic responses and ganglion cell counts. Cell therapies for LHON therefore represent a promising therapeutic approach, and may be of particular utility in treating more advanced disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yu-Wai-Man P, Griffiths PG, Hudson G et al: Inherited mitochondrial optic neuropathies. J Med Genet 2009; 46: 145–158.
Farrar GJ, Chadderton N, Kenna PF et al: Mitochondrial disorders: aetiologies, models systems, and candidate therapies. Trends Genet 2013; 29: 488–497.
Chadderton N, Palfi A, Millington-Ward S et al: J. Intravitreal delivery of AAV-NDI1 provides functional benefit in a murine model of Leber hereditary optic neuropathy. Eur J Hum Genet 2013; 21: 62–68.
Yu-Wai-Man P, Griffiths PG, Chinnery PF : Mitochondrial optic neuropathies- disease mechanisms and therapeutic strategies. Prog Retin Eye Res 2011; 30: 81–114.
Lin CS, Sharpley MS, Fan W : Mouse mtDNA mutant model of Leber hereditary optic neuropathy. Proc Natl Acad Sci USA 2012; 109: 20065–20070.
Alexander C, Votruba M, Pesch UE et al: OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 2000; 26: 211–215.
Aung T, Ocaka L, Ebenezer ND et al: A major marker for normal tension glaucoma: association with polymorphisms in the OPA1 gene. Hum Genet 2002; 110: 52–56.
Lee S, Sheck L, Crowston JG et al: Impaired complex-I-linked respiration and ATP synthesis in primary open-angle glaucoma patient lymphoblasts. Invest Ophthalmol Vis Sci 2012; 53: 2431–2437.
Zhang X, Jones D, Gonzalez-Lima F : Mouse model of optic neuropathy caused by mitochondrial complex I dysfunction. Neurosci Lett 2002; 326: 97–100.
Zhang X, Jones D, Gonzalez-Lima F : Neurodegeneration produced by rotenone in the mouse retina: a potential model to investigate environmental pesticide contributions to neurodegenerative diseases. J Toxicol Environ Health A 2006; 69: 1681–1697.
Hayworth CR, Rojas JC, Gonzalez-Lima F : Transgenic mice expressing cyan fluorescent protein as a reporter strain to detect the effects of rotenone toxicity on retinal ganglion cells. J Toxicol Environ Health A 2008; 71: 1582–1592.
Bartsch U, Oriyakhel W, Kenna PF et al: Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res 2008; 86: 691–700.
West E, Pearson R, Duran Y et al: Manipulation of the recipient retinal environment by ectopic expression of neurotrophic growth factors can improve transplanted photoreceptor integration and survival. Cell Transplant 2012; 21: 871–887.
Mansergh FC, Vawda R, Millington-Ward S et al: Loss of photoreceptor potential from retinal progenitor cell cultures, despite improvements in survival. Exp Eye Res 2010; 91: 500–512.
Mansergh F, Orton NC, Vessey JP et al: Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet 2005; 14: 3035–3046.
Kiang S, Palfi A, Ader M et al: Toward a gene therapy for dominant disease; validation of an RNA interference based mutation independent approach. Mol Ther 2005; 12: 555–561.
Pautler RG : Biological applications of manganese-enhanced magnetic resonance imaging. Methods Mol Med 2006; 124: 365–386.
MacLaren RE, Pearson RA, MacNeil A et al: Retinal repair by transplantation of photoreceptor precursors. Nature 2006; 444: 203–207.
Eberle D, Kurth T, Santos-Ferreira T et al: Outer segment formation of transplanted photoreceptor precursor cells. PLoS One 2012; 7: e46305.
Wallace VA : Concise review: making a retina—from the building blocks to clinical applications. Stem Cells 2011; 29: 412–417.
Czekaj M, Haas J, Gebhardt M et al: In vitro expanded stem cells from the developing retina fail to generate photoreceptors but differentiate into myelinating oligodendrocytes. PLoS One 2012; 7: e41798.
Richter F, Meurers BH, Zhu C et al: Neurons express hemoglobin alpha- and beta-chains in rat and human brains. J Comp Neurol 2009; 515: 538–547.
Tezel G, Yang X, Luo C et al: Hemoglobin expression and regulation in glaucoma: insights into retinal ganglion cell oxygenation. Invest Ophthalmol Vis Sci 2010; 51: 907–919.
Mansergh FC, Hunter SM, Geatrell JC et al: Developmentally regulated expression of hemoglobin subunits in avascular tissues. Int J Dev Biol 2008; 52: 873–886.
West EL, Pearson RA, Barker SE et al: Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells 2010; 28: 1997–2007.
O'Brien T, Barry FP : Stem cell therapy and regenerative medicine. Mayo Clin Proc 2009; 84: 859–861.
Ratajczak MZ, Kucia M, Jadczyk T et al: Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012; 26: 1166–1173.
Manuguerra-Gagné R, Boulos PR, Ammar A et al: Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells 2013; 31: 1136–1148.
FitzGibbon T, Nestorovski Z : Morphological consequences of myelination in the human retina. Exp Eye Res 1997; 65: 809–819.
Duval R, Hammamji K, Aroichane M et al: Acquired myelinated nerve fibers in association with optic disk drusen. J AAPOS 2010; 14: 544–547.
Stieger K, Cronin T, Bennett J, Rolling F : Adeno-associated virus mediated gene therapy for retinal degenerative diseases. Methods Mol Biol 2011; 807: 179–218.
Ellouze S, Augustin S, Bouaita A et al: Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet 2008; 83: 373–387.
Yu H, Koilkonda RD, Chou TH et al: Gene delivery to mitochondria by targeting modified adeno-associated virus suppresses Leber's hereditary optic neuropathy in a mouse model. Proc Natl Acad Sci USA 2012; 109: E1238–E1247.
Acknowledgements
We would like to thank the staff of the Bioresources Unit, Trinity College Dublin, for technical assistance, Rustam Rakhmatullin for technical assistance with MEMRI and Dr Michael Wride for helpful discussion and critical reading of this manuscript. We would also like to thank Don Zack for his gift of the eGFP transgenic mice. This research was funded by Fighting Blindness Ireland, the Health Research Board of Ireland (HRB) and Science Foundation Ireland (SFI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Professor Farrar and Dr Kenna are directors of Genable Technologies; Dr Chadderton is a consultant for Genable Technologies, Dr Mansergh and Dr Gobbo declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mansergh, F., Chadderton, N., Kenna, P. et al. Cell therapy using retinal progenitor cells shows therapeutic effect in a chemically-induced rotenone mouse model of Leber hereditary optic neuropathy. Eur J Hum Genet 22, 1314–1320 (2014). https://doi.org/10.1038/ejhg.2014.26
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2014.26
This article is cited by
-
Ubiquinone binding site of yeast NADH dehydrogenase revealed by structures binding novel competitive- and mixed-type inhibitors
Scientific Reports (2018)
-
The retina as an early biomarker of neurodegeneration in a rotenone-induced model of Parkinson’s disease: evidence for a neuroprotective effect of rosiglitazone in the eye and brain
Acta Neuropathologica Communications (2016)