Abstract
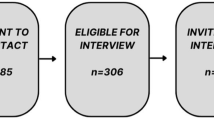
Population-based genetic research may produce information that has clinical implications for participants and their family. Researchers notify participants or their next of kin (NoK) about the availability of genetic information via a notification letter; however, many subsequently do not contact a family cancer centre (FCC) to clarify their genetic status. Therefore, the purpose of this study was to examine research participants’ experience of receiving a notification letter and the factors that influenced contact with an FCC. Twenty-five semi-structured interviews were conducted with research participants (n=10) or their NoK (n=15) who had received a notification letter following participation in the Australian Ovarian Cancer Study. There were a number of factors which impacted participants’ access to genetic counselling at an FCC. Some participants had unmet information and support needs, which were addressed by their participation in this psychosocial interview study. Recruitment and participation in this study therefore inadvertently increased a number of participants’ intention to contact an FCC. For others, participation in this study facilitated access to an FCC. Recommendations are proposed regarding future notification as well as implications for clinical practice. An approach that also provides opportunity to address research participants’ support and informational needs before contacting a clinical genetics service as well as practical guidance for accessing genetic services would facilitate timely and smooth access for research participants who are interested in following up clinically relevant genetic test results.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Knoppers BM, Joly Y, Simard J, Durocher F : The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet 2006; 14: 1170–1178.
Quaid KA, Jessup NM, Meslin EM : Disclosure of genetic information obtained through research. Genet Test 2004; 8: 347–355.
National Health and Medical Research Council: National Statement on Ethical Conduct in Research Involving Humans. Canberra: Commonwealth of Australia, 2007.
Richards MP, Ponder M, Pharoah P, Everest S, Mackay J : Issues of consent and feedback in a genetic epidemiological study of women with breast cancer. J Med Ethics 2003; 29: 93–96.
Murphy J, Scott J, Kauffman D, Gellar G, Leroy L, Hudson K : Public expectations for return of results from large-cohort genetic research. Am J Bioeth 2008; 8: 36–43.
Domchek SM, Friebel TM, Singer CF et al: Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010; 304: 967–975.
Rebbeck TR, Kauff ND, Domchek SM : Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst 2009; 101: 80–87.
Keogh LA, Fisher D, Sheinfeld Gorin S et al: How do researchers manage genetic results in practice? The experience of the multinational Colon Cancer Family Registry. J Community Genet 2013; 5: 99–108.
Keogh LA, Southey MC, Maskiell J et al: Uptake of offer to receive genetic information about BRCA1 and BRCA2 mutations in an Australian population-based study. Cancer Epidemiol Biomarkers Prev 2004; 13: 2258–2263.
Wakefield CE, Thorne H, Kirk J et al: Improving mutation notification when new genetic information is identified in research: a trial of two strategies in familial breast cancer. Genet Med 2013; 15: 187–194.
Wakefield CE, Thorne H, Kirk J et al: ‘For all my family’s sake, I should go and find out’: An Australian Report on Genetic Counseling and Testing Uptake in Individuals at High Risk of Breast and/or Ovarian Cancer. Genet Test Mol Biomarkers 2011; 15: 379–385.
Keogh LA, Vliet C, Studdert D et al: Is uptake of genetic testing for colorectal cancer influenced by knowledge of insurance implications? Med J Aust 2009; 191: 255–258.
Keogh LA, McClaren B, Maskiell J et al: How do individuals decide whether to accept or decline an offer of genetic testing for colorectal cancer? Ann Behav Med 2011; 41: S129–S129.
Hallowell N, Alsop K, Gleeson M et al: The responses of research participants and their next of kin to receiving feedback of genetic test results following participation in the Australian Ovarian Cancer Study. Genet Med 2013; 15: 458–465.
Strauss A, Corbin J : Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Newbury Park: Sage Publications, 1990.
Alsop K, Fereday S, Meldrum C et al: BRCA mutation frequency and patterns of treatment response in BRCA mutation positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 2012; 30: 2654–2663.
Walter FM, Emery J : ‘Coming down the line’—patients’ understanding of their family history of common chronic disease. Ann Fam Med 2005; 3: 405–414.
Clayton EW, McGuire AL : The legal risks of returning results of genomics research. Genet Med 2012; 14: 473–477.
Young MA : Ethical tensions in genetic counselling research. Monash Bioeth Rev 2011; 29: 1–12.
Roberts JS, Shalowitz D, Christensen K et al: Returning individual research results: development of a cancer genetics education and risk communication protocol. J Empir Res Hum Res Ethics 2010; 5: 17–30.
Christensen KD, Roberts JS, Shalowitz DI et al: Disclosing individual CDKN2A research results to melanoma survivors: interest, impact, and demands on researchers. Cancer Epidemiol Biomarkers Prev 2011; 20: 522–529.
Acknowledgements
We thank all the women who participated in AOCS, particularly the women and their relatives who took part in the psychosocial study. AOCS was supported by the US Army Medical Research and Materiel Command under DAMD17-01-1-0729, The Cancer Council Victoria, Queensland Cancer Fund, The Cancer Council New South Wales, The Cancer Council South Australia, The Cancer Foundation of Western Australia, The Cancer Council Tasmania and the National Health and Medical Research Council of Australia (NHMRC; ID400413, ID400281). The full study group can be found at www.aocstudy.org. The AOCS prevalence study was supported by the Ovarian Cancer Research Program of the US Department of Defense (W81XWH-08-1-0684 and W81XWH-08-1-0685), Cancer Australia (509303), the Cancer Council Victoria and the Peter MacCallum Cancer Centre Foundation. We gratefully acknowledge the cooperation of the participating institutions in Australia and also the contribution of the study nurses, research assistants and all clinical and scientific collaborators. The complete AOCS study group can be found at www.aocstudy.org.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Crook, A., Plunkett, L., Forrest, L. et al. Connecting patients, researchers and clinical genetics services: the experiences of participants in the Australian Ovarian Cancer Study (AOCS). Eur J Hum Genet 23, 152–158 (2015). https://doi.org/10.1038/ejhg.2014.86
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2014.86
This article is cited by
-
My Research Results: a program to facilitate return of clinically actionable genomic research findings
European Journal of Human Genetics (2022)
-
Oncologists’ perspectives of telephone genetic counseling to facilitate germline BRCA1/2 testing for their patients with high-grade serous ovarian cancer
Journal of Community Genetics (2021)
-
Evaluation of telephone genetic counselling to facilitate germline BRCA1/2 testing in women with high-grade serous ovarian cancer
European Journal of Human Genetics (2019)
-
Development and Pilot Testing of a Decision Aid for Genomic Research Participants Notified of Clinically Actionable Research Findings for Cancer Risk
Journal of Genetic Counseling (2018)