Abstract
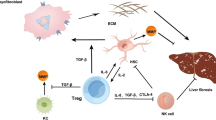
Cells termed myofibroblasts are prominent in the injury response of all epithelial tissues. They exhibit proliferation, migration, production of collagen and other extracellular matrix (ECM) molecules, and contraction, all for containing the injury and closing the wound. When the injury is limited in time, the final stage of the repair involves a dismantling of the cellular apparatus and restoration of normal tissue structure. With multiple cycles of repair, however, there is net accumulation of ECM, to the detriment of tissue structure and function. Repair-related ECM coalesces into fibrous bundles and, over time, undergoes changes that render it resistant to degradation. The result is a scar. In the skin, a scar may have cosmetic importance only. In the liver, however, extensive scarring is the setting for unregulated growth and neoplasia; also, fibrous bands disrupt normal blood flow, leading to portal hypertension and its complications. With regard to therapy for fibrosis, the first consideration is elimination of the injury factor. However, given that many liver diseases do not have effective therapies at present, strategies targeting fibrogenesis per se are under development. The main source of myofibroblast-like cells and ECM production in the liver is the perisinusoidal stellate cell, which responds to injury with a pleiotypic change termed activation. Activation is orchestrated by cytokines and the ECM itself. Among the cytokines involved in this process, transforming growth factor-β (TGF-β) is particularly prominent. The early changes in ECM include de novo production of a specific "fetal" isoform of fibronectin, which arises from sinusoidal endothelial cells. It is stimulated by TGF-β and acts directly on stellate cells to promote their activation. Based on these and other advances in understanding the fundamentals of the injury response, several strategies now exist for altering fibrogenesis, ranging from agents that block TGF-β to traditional Chinese herbal extracts. Arrest of fibrogenesis, even with underlying cirrhosis, is likely to extend life or prolong the time to transplant. Whether it reduces the risk of hepatocellular carcinoma remains to be proven. Although TGF-β antagonists are effective anti-fibrogenic agents, they will require detailed safety testing because of the finding that several forms of epithelial neoplasia are associated with altered regulation of TGF-β.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bissell, D. Chronic liver injury, TGF-β, and cancer. Exp Mol Med 33, 179–190 (2001). https://doi.org/10.1038/emm.2001.31
Published:
Issue date:
DOI: https://doi.org/10.1038/emm.2001.31
Keywords
This article is cited by
-
Comparison of Extracellular Matrix (ECM) of Normal and D-Galactosamine-Induced Mice Model of Liver Injury Before and After Liver Decellularization
Regenerative Engineering and Translational Medicine (2021)
-
The hypoxic tumour microenvironment
Oncogenesis (2018)
-
Galunisertib modifies the liver fibrotic composition in the Abcb4Ko mouse model
Archives of Toxicology (2018)
-
Hepatic inflammation-fibrosis-cancer axis in the rat hepatocellular carcinoma induced by diethylnitrosamine
Journal of Cancer Research and Clinical Oncology (2017)
-
Cell biology is different in small volumes: endogenous signals shape phenotype of primary hepatocytes cultured in microfluidic channels
Scientific Reports (2016)