Abstract
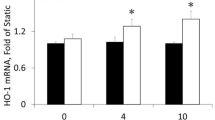
Oxidative stress has been implicated in mediation of vascular disorders. In the presence of vanadate, H2O2 induced tyrosine phosphorylation of PLD1, protein kinase C-α (PKC-α), and other unidentified proteins in rat vascular smooth muscle cells (VSMCs). Interestingly, PLD1 was found to be constitutively associated with PKC-α in VSMCs. Stimulation of the cells by H2O2 and vanadate showed a concentration-dependent tyrosine phosphorylation of the proteins in PLD1 immunoprecipitates and activation of PLD. Pretreatment of the cells with the protein tyrosine kinase inhibitor, genistein resulted in a dose-dependent inhibition of H2O2-induced PLD activation. PKC inhibitor and down-regulation of PKC abolished H2O2-stimulated PLD activation. The cells stimulated by oxidative stress (H2O2) caused increased cell migration. This effect was prevented by the pretreatment of cells with tyrosine kinase inhibitors, PKC inhibitors, and 1-butanol, but not 3-butanol. Taken together, these results suggest that PLD might be involved in oxidative stress-induced migration of VSMCs, possibly via tyrosine phosphorylation and PKC activation.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kim, J., Min, G., Bae, YS. et al. Phospholipase D is involved in oxidative stress-induced migration of vascular smooth muscle cells via tyrosine phosphorylation and protein kinase C. Exp Mol Med 36, 103–109 (2004). https://doi.org/10.1038/emm.2004.15
Published:
Issue date:
DOI: https://doi.org/10.1038/emm.2004.15
Keywords
This article is cited by
-
Mechanical properties of the extracellular matrix alter expression of smooth muscle protein LPP and its partner palladin; relationship to early atherosclerosis and vascular injury
Journal of Muscle Research and Cell Motility (2009)