Abstract
Purpose
This pilot study was undertaken to examine the relationships between clinical measures of visual function and anatomic changes occurring in the eyes treated with bevacizumab for choroidal neovascularization (CNV) due to age-related macular degeneration (AMD).
Methods
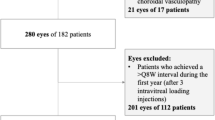
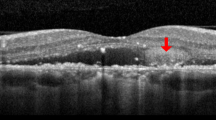
A retrospective review was conducted for 50 eyes that had been treated with at least three injections of bevicizumab for CNV due to AMD, and followed for at least 6 months. Vision outcomes included best-corrected ETDRS chart acuity, scored by best-line read (ETDRS line) and by total letters read (ETDRS letter), and two measures obtained from central acuity perimetry with 98% Michelson contrast targets, the best acuity within 6° of fixation (BA6°), and global macular acuity (GMA), representing a weighted average of the acuities thresholded at all intercepts within a 10° radius of fixation. Assessment of anatomic outcomes included fibrosis, atrophy, and subretinal hemorrhage grading on fundus photography, CNV size, pigment epithelial detachment (PED) size and grading of CNV leakage on fluorescein angiography, and central retinal PED, and subretinal fluid (SRF) thickness on optical coherence tomography.
Results
Logistic regression analysis showed an association between the vision outcomes of EDTRS letter and BA6° with the change in SRF thickness (R2: 0.47 and 0.35, respectively). The outcome of the vision measurement of GMA was associated with the change in SRF thickness, in CNV thickness, and in CNV fibrosis grade (R2: 0.34). No association was noted between the outcomes of ETDRS line with the change in any anatomic outcomes.
Conclusion
Acuity perimetry outcomes in this study seemed to offer improved understanding of the relationship between the vision outcomes and the measured anatomic changes. It seemed that neither ocular coherence tomography nor fluorescein angiography alone offered sufficient morphologic markers for prediction of functional outcomes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Klein R, Klein BE, Jensen SC, Meuer SM . The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997; 104: 7–21.
Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ . Intravitreal bevacizumab (Avastin) for neovascular age-related macular dejeneration. Ophthalmology 2006; 113: 363–372.
Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN . Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular dejeneration. Am J Ophthalmol 2006; 142: 1–9.
Augustin AJ, Puls S, Offermann I . Triple therapy for choroidal neovascularization due to age-related macular degeneration: verteporfin PDT, bevacizumab, and dexamethasone. Retina 2007; 27: 133–140.
Krebs I, Binder S, Stolba U, Schmid K, Glittenberg C, Brannath W et al. Optical coherence tomography guided retreatment of photodynamic therapy. Br J Ophthalmol 2005; 89: 1184–1187.
Sahni J, Stanga P, Wong D, Harding S . Optical coherence tomography in photodynamic therapy for subfoveal choroidal neovascularisation secondary to age related macular degeneration: a cross sectional study. Br J Ophthalmol 2005; 89: 316–320.
Moutary T, Alarbi M, Mahon G, Stevenson M, Chakravarthy U . Relationships between clinical measures of visual function, fluorescein angiographic and optical coherence tomography features in patients with subfoveal choroidal neovascularization. Br.J. Ophthalmol 2008; 92: 361–364.
Hee MR, Baumal CR, Puliafito CA, Duker JS, Reichel E, Wilkins JR et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology 1996; 10: 1260–1270.
Chen CY, Wong JY, Heriot WJ et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration: a short-term study. Am J Ophthalmol 2007; 143: 510–512.
Yoganathan P, Deramo VA, Lai JC, Tibrewala RK, Fastenberg DM . Visual improvement following intravitreal bevacizumab (Avastin) in exudative age-related macular degeneration. Retina 2006; 26: 994–998.
Salinas-Alaman A, Garcia-Layana A, Maldonado MJ, Sainz-Gómez C, Alvárez-Vidal A . Using optical coherence tomography to monitor photodynamic therapy in age-related macular degeneration. Am J Ophthalmol 2005; 140: 23–28.
Ting TD, Oh M, Cox TA, Meyer CH, Toth CA . Decreased visual acuity associated with cystoid macular edema in neovascular age-related macular degeneration. Arch Ophthalmol 2002; 120: 731–737.
Sinclair SH, Alaniz R, Presti P . Laser treatment of diabetic macular edema: comparison of ETDRS-level treatment with threshold-level treatment by using high-contrast discriminant central visual field testing. Semin Ophthalmol 1999; 14 (4): 214–222.
Ferris III FL, Kassoff A, Bresnick GH, Bailey I . New visual acuity charts for clinical research. Am J Ophthalmol 1982; 94: 91–96.
Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP et al. Optical coherence tomography of the human retina. Arch Ophthalmol 1995; 113: 325–332.
Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT . Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 1997; 81: 154–162.
Zarbin MA . Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 2004; 122: 598–614.
Kliffen M, Van der Schaft T, Mooy CM, De Jong PT . Morphologic changes in age-related maculopathy. Microsc Res Tech 1997; 36: 106–122.
Gonzalez E, Tarita-Nistor L, Markowitz SN, Steinbach MJ . Computer-based test to measure optimal visual acuity in age-related macular degeneration. Invest Ophthalmol Visl Sci 2007; 48: 4838–4845.
Moore T . Shape representations and visual guidance of saccadic eye movements. Science 1999; 285: 5435.
Hoffman JE . The role of attention in saccadic eye movements. Percept Psychophys 1995; 5: 787–795.
Vogel GL . Saccadic eye movements: theory testing and therapy. J Behav Optom 1995; 6 (1): 3–12.
Sunness J, Applegate C, Haselwood D, Rubin GS . Fixation patterns and rading rates in eyes with central scotomas from advanced atrophic age-related macular degeneration and Stargardt disease. Ophthalmology 1996; 103: 1458–1466.
Sunness J, Applegate C . Long-term follow-up of fixation patterns in eyes with central scotomas from geographic trophy that is associated with age-related macular degeration. Am J Ophthalmol 2005; 140: 1085–1093.
Spaide RF, Laud K, Fine HF, Klancnik Jr JM, Meyerle CB, Yannuzzi LA et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina 2006; 26: 383–390.
Aggio FB, Farah ME, Silva WC, Melo GB . Intravitreal bevacizumab for exudative age-related macular degeneration after multiple treatments. Graefes Arch Clin Exp Ophthalmol 2007; 245: 215–220.
Rich RM, Rosenfeld PJ, Puliafito CA, Dubovy SR, Davis JL, Flynn Jr HW et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina 2006; 26: 495–511.
Emerson MV, Lauer AK, Flaxel CJ, Wilson DJ, Francis PJ, Stout JT et al. Intravitreal bevacizumab (Avastin) treatment of neovascular age-related macular degeneration. Retina 2007; 27: 439–444.
Lazic R, Gabric N . Intravitreally administered bevacizumab (Avastin) in minimally classic and occult choroidal neovascularization secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2007; 245: 68–73.
Pedersen R, Soliman W, Lund-Andersen H, Larsen M . Treatment of choroidal neovascularization using intravitreal bevacizumab. Acta Ophthalmol Scand 2007; 85: 526–533.
Jonas JB, Libondi T, Ihloff AK, Harder B, Kreissig I, Schlichtenbrede F et al. Visual acuity change after intravitreal bevacizumab for exudative agerelated macular degeneration in relation to subfoveal membrane type. Acta Ophthalmol Scand 2007; 85: 563–565.
Aisenbrey S, Ziemssen F, Völker M, Gelisken F, Szurman P, Jaissle G et al. Intravitreal bevacizumab (Avastin) for occult choroidal neovascularization in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2007; 247: 941–948.
Abraham-Marin ML, Cortes-Luna CF, Alvarez-Rivera G, Hernández-Rojas M, Quiroz-Mercado H, Morales-Cantón V . Intravitreal bevacizumab therapy for neovascular age-related macular degeneration: a pilot study. Graefes Arch Clin Exp Ophthalmol 2007; 245: 651–655.
Levy J, Shneck M, Rosen S, Klemperer I, Rand D, Weinstein O et al. Intravitreal bevacizumab (avastin) for subfoveal neovascular age-related macular degeneration. Int Ophthalmol 2008. [Epub ahead of print].
Acknowledgements
This study was supported by TUBITAK.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was presented in 2008 ARVO meeting.
Rights and permissions
About this article
Cite this article
Ünver, Y., Yavuz, G., Bekiroğlu, N. et al. Relationships between clinical measures of visual function and anatomic changes associated with bevacizumab treatment for choroidal neovascularization in age-related macular degeneration. Eye 23, 453–460 (2009). https://doi.org/10.1038/eye.2008.349
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2008.349
Keywords
This article is cited by
-
Fluid as a critical biomarker in neovascular age-related macular degeneration management: literature review and consensus recommendations
Eye (2021)
-
Structures affecting recovery of macular function in patients with age-related macular degeneration after intravitreal ranibizumab
Graefe's Archive for Clinical and Experimental Ophthalmology (2015)
-
Recovery of photoreceptor outer segments after anti-VEGF therapy for age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2013)
-
Intravitreal bevacizumab (Avastin) for age-related macular degeneration: a critical analysis of literature
Eye (2010)