Abstract
Purpose
To evaluate the safety and efficacy of intravitreal bevacizumab in Chinese patients with choroidal neovascularization (CNV) secondary to pathologic myopia.
Methods
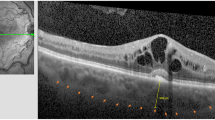
Eight eyes of consecutive patients with myopic CNV without earlier treatment were treated with intravitreal injection of bevacizumab (2.5 mg). All patients underwent a clinical examination, which included visual acuity measurements, fundus photography, fluorescein angiography, and optical coherence tomography.
Results
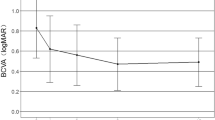
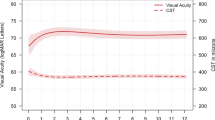
Eight eyes of eight patients with ages ranging from 26 to 62 years (mean 41.5 years) were enroled. The follow-up period ranged from 13 to 17 months (mean, 14.9 months). At the 12-month follow-up, vision had improved in all eyes by three or more lines. However, a new-onset CNV adjacent to the earlier lesion and visual loss of one line at the 16th month were observed in one eye. The mean VA had significantly improved from the baseline value 20/82 to 20/25 (P=0.017). The final mean central retinal thickness was 214.1±36.3 μm, with a mean decrease of 59.8 μm from the pre-treatment value (P=0.017). The mean number of injections was 1.4 (ranging from 1 to 2) within 12 months. No other ocular or systemic side effects were noted.
Conclusions
In this small series of patients with 1-year follow-up, intravitreal injection of 2.5 mg bevacizumab seems to be effective and safe in patients with myopic CNV.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ . Etiology of choroidal neovascularization in young patients. Ophthalmology 1996; 103 (8): 1241–1244.
Tabandeh H, Flynn Jr HW, Scott IU, Lewis ML, Rosenfeld PJ, Rodriguez F et al. Visual acuity outcomes of patients 50 years of age and older with high myopia and untreated choroidal neovascularization. Ophthalmology 1999; 106 (11): 2063–2067.
Yoshida T, Ohno-Matsui K, Ohtake Y, Takashima T, Futagami S, Baba T et al. Long-term visual prognosis of choroidal neovascularization in high myopia: a comparison between age groups. Ophthalmology 2002; 109 (4): 712–719.
Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, Lewis H et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial—VIP report no. 3. Ophthalmology 2003; 110 (4): 667–673.
Wu P, Chen Y, Chen C, Chen YH, Kao ML, Shin SJ et al. Subthreshold transpupillary thermotherapy in Chinese patients with myopic choroidal neovascularization: 1 and 2 year follow-up. Clin Exp Ophthalmol 2008; 36 (5): 443–448.
Chan WM, Lai TY, Liu DT, Lam DS . Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: six-month results of a prospective pilot study. Ophthalmology 2007; 114 (12): 2190–2196.
Hernandez-Rojas ML, Quiroz-Mercado H, Dalma-Weiszhausz J, Fromow-Guerra J, Amaya-Espinosa A, Solís-Vivanco A et al. Short-term effects of intravitreal bevacizumab for subfoveal choroidal neovascularization in pathologic myopia. Retina 2007; 27 (6): 707–712.
Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB . Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results. Am J Ophthalmol 2009; 147 (1): 84–93.
Ikuno Y, Sayanagi K, Soga K, Sawa M, Tsujikawa M, Gomi F et al. Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol 2009; 147 (1): 94–100.
Costa RA, Jorge R, Calucci D, Cardillo JA, Melo Jr LA, Scott IU . Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci 2006; 47 (10): 4569–4578.
Yoganathan P, Fastenberg DM, Ferrone PJ . Intravitreal Bevacizumab 2.5 versus 1.25 mg in Exudative Age-Related Macular Degeneration: Safety and Efficacy. Program and Abstracts of the Association for Research in Vision and Ophthalmology. Fort Lauderdale: Florida, 2007 (Abstract 166).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest: none.
Rights and permissions
About this article
Cite this article
Wu, PC., Chen, YJ. Intravitreal injection of bevacizumab for myopic choroidal neovascularization: 1-year follow-up. Eye 23, 2042–2045 (2009). https://doi.org/10.1038/eye.2008.404
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2008.404
Keywords
This article is cited by
-
Recent trends in the management of maculopathy secondary to pathological myopia
Graefe's Archive for Clinical and Experimental Ophthalmology (2012)
-
Prognostic factors for visual outcomes 2-years after intravitreal bevacizumab for myopic choroidal neovascularization
Eye (2011)
-
Myopic choroidal neovascularization treated by intravitreal bevacizumab: comparison of two different initial doses
Graefe's Archive for Clinical and Experimental Ophthalmology (2011)
-
Intravitreal bevacizumab for choroidal neovascularisation secondary to causes other than age-related macular degeneration
Eye (2010)
-
Bevacizumab for choroidal neovascularization secondary to pathologic myopia: Is there a decline of the treatment efficacy after 2 years?
Graefe's Archive for Clinical and Experimental Ophthalmology (2010)