Abstract
Background
Although randomized clinical trials (ANCHOR and MARINA) have shown excellent results of ranibizumab treatment in patients with neovascular age-related macular degeneration (AMD), it is unclear whether such an outcome is achievable in daily practice. We evaluated the results of ranibizumab treatment for neovascular AMD in clinical practice in Australia.
Methods
A retrospective chart review of patients in four practices injected with ranibizumab in 2006 for AMD. Patients who had been diagnosed with subfoveal choroidal neovascular membrane in the preceding 6 months and had completed at least 6 months follow-up were enrolled. No standard treatment protocols were required. The main outcome measure was visual acuity (VA) at 6 and 12 months.
Results
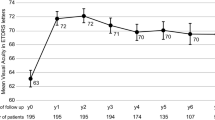
A total of 158 patients fulfilled the entry criteria. The mean baseline VA (decimal) was 0.35±0.21 (Snellen equivalent 6/17). At 6 months, the mean VA improved to 0.46±0.27 (6/13) and remained stable until month 12 (0.48±0.30). The improvement in VA between baseline and months 6 and 12 was statistically significant (P<0.0001). Both the mean and the median number of injections were four in the first 6 months and nine at 12 months. VA results were comparable with those of the ANCHOR and MARINA trials, and were achieved with a lower number of injections (P<0.0001).
Conclusion
VA results achieved in daily clinical practice using ranibizumab for neovascular AMD are similar to large prospective randomized trials.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab vs verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1432–1444.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1419–1431.
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: pier study year 1. Am J Ophthalmol 2008; 145 (2): 239–248 e235.
Bressler NM, Bressler SB, Fine SL . Age-related macular degeneration. Surv Ophthalmol 1988; 32 (6): 375–413.
VanNewkirk MR, Nanjan MB, Wang JJ, Mitchell P, Taylor HR, McCarty CA . The prevalence of age-related maculopathy: the visual impairment project. Ophthalmology 2000; 107 (8): 1593–1600.
Ferris III FL, Fine SL, Hyman L . Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984; 102 (11): 1640–1642.
Lucentis FDA official information, side-effects and uses. Available athttp://www.drugs.com/pro/lucentis.html. Accessed on 12 Feb 2008.
Kotter I, Zierhut M, Eckstein AK, Vonthein R, Vonthein R, Ness T et al. Human recombinant interferon alfa-2a for the treatment of Behcet's disease with sight threatening posterior or panuveitis. Br J Ophthalmol 2003; 87 (4): 423–431.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007; 143 (4): 566–583.
Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first year ANCHOR results. Am J Ophthalmol 2007; 111: 850–857.
Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR, for the MARINA study group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2007; 114: 246–252.
Alexander SL, Linde-Zwirble WT, Werther W, Depperschmidt EE, Wilson LJ, Palanki R et al. Annual rates of arterial thromboembolic events in medicare neovascular age-related macular degeneration patients. Ophthalmology 2007; 114 (12): 2174–2178.
Acknowledgements
Novartis provided financial support to help the Centre for Eye Research Australia to conduct this study. The data analysis was performed independently by Datapharm, Drummoyne, New South Wales. Novartis had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 40th Annual Scientific Congress of The Royal Australian and New Zealand College of Ophthalmologists, Melbourne, Australia, 2008
Financial disclosure: K Michalova's and S Wickremasinghe's fellowships at the Centre for Eye Research Australia were supported by Novartis. RH Guymer, CA Harper, and AP Hunyor are on the medical retina advisory board of Novartis Australia. RH Guymer and A Chang were principal investigators in Novartis-sponsored trials in AMD.
Rights and permissions
About this article
Cite this article
Michalova, K., Wickremasinghe, S., Tan, T. et al. Ranibizumab treatment for neovascular age-related macular degeneration: from randomized trials to clinical practice. Eye 23, 1633–1640 (2009). https://doi.org/10.1038/eye.2009.175
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2009.175
Keywords
This article is cited by
-
A systematic approach to evaluate practice-based process- and outcome data applied to the treatment of neovascular age-related macular degeneration
BMC Ophthalmology (2020)
-
Which visual acuity measurements define high-quality care for patients with neovascular age-related macular degeneration treated with ranibizumab?
Eye (2013)
-
Neovascular age-related macular degeneration: decision making and optimal management
Eye (2010)