Abstract
Purpose
The purpose of this study was to describe the long-term results of AlphaCor implantations, and to evaluate the main complications and risk factors.
Methods
Retrospective analysis of preoperative and follow-up data from 15 AlphaCor implantations. Analysis of outcomes, trends, and associations was performed and compared with data from published clinical trials and a literature review.
Results
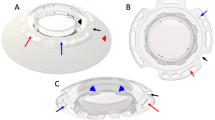
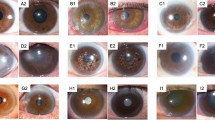
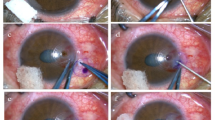
The survival rate of the device at 1, 2, and 3 years was 87%, 58%, and 42%, respectively. Postoperative visual acuity ranged from hand movement to 0.8. The most significant complications were stromal melt (nine cases), optic deposition (three eyes), and retroprosthetic membrane formation (three eyes). The most common device-unrelated complication was trauma (three patients). All complications were managed without loss of the eye.
Conclusion
AlphaCor provides a treatment option for patients with corneal blindness in which a donor tissue graft would not succeed.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hicks CR, Crawford GJ . Indications and technique: AlphaCor artificial cornea. Tech Ophthalmol 2003; 1: 151–155.
Hicks CR, Crawford GJ, Dart JK, Grabner G, Holland EJ, Stulting RD et al. AlphaCor clinical outcomes. Cornea 2006; 25: 1034–1042.
Chirila TV, Vijayasekaran S, Horne R, Chen YC, Dalton PD, Constable IJ et al. Interpenetrating polymer network (IPN) as a permanent joint between the elements of a new type of artificial cornea. J Biomed Mater Res 1994; 28: 745–753.
Hicks CR, Crawford GJ, Tan DT, Snibson GR, Sutton GL, Downie N et al. AlphaCor cases: comparative outcomes. Cornea 2003; 22: 583–590.
Chirila TV, Thompson-Wallis DE, Crawford GJ, Constable IJ, Vijayasekaran S . Production of neocollagen by cells invading hydrogel sponges implanted in the rabbit cornea. Graefes Arch Clin Exp Ophthalmol 1996; 234: 193–198.
Crawford GJ, Constable IJ, Chirila TV, Vijayasekaran S, Thompson DE . Tissue interaction with hydrogel sponges implanted in the rabbit cornea. Cornea 1993; 12: 348–357.
Crawford GJ, Chirila TV, Vijayasekaran S, Dalton PD, Constable IJ . Preliminary evaluation of a hydrogel core-and-skirt keratoprosthesis in the rabbit cornea. J Refract Surg 1996; 12: 525–529.
Fitton JH, Ziegelaar BW, Hicks CR, Clayton AB, Crawford GJ, Constable IJ et al. Assessment of anticollagenase treatments after insertion of a keratoprosthetic material in the rabbit cornea. Cornea 1998; 17: 108–114.
Hicks CR, Chirila TV, Clayton AB, Fitton JH, Vijayasekaran S, Dalton PD et al. Clinical results of implantation of the Chirila keratoprosthesis in rabbits. Br J Ophthalmol 1998; 82: 18–25.
Hicks CR, Chirila TV, Dalton PD, Clayton AB, Vijayasekaran S, Crawford GJ et al. Keratoprosthesis: preliminary results of an artificial corneal button as a full-thickness implant in the rabbit model. Aust NZ J Ophthalmol 1996; 24: 297–303.
Hicks CR, Fitton JH, Chirila TV, Crawford GJ, Constable IJ . Keratoprostheses: advancing toward a true artificial cornea. Surv Ophthalmol 1997; 42: 175–189.
Hicks CR, Lou X, Platten S, Clayton AB, Vijayasekaran S, Fitton HJ et al. Keratoprosthesis results in animals: an update. Aust NZ J Ophthalmol 1997; 25 (Suppl 1): S50–S52.
Hicks CR, Vijayasekaran S, Chirila TV, Platten ST, Crawford GJ, Constable IJ . Implantation of PHEMA keratoprostheses after alkali burns in rabbit eyes. Cornea 1998; 17: 301–308.
Vijayasekaran S, Fitton JH, Hicks CR, Chirila TV, Crawford GJ, Constable IJ . Cell viability and inflammatory response in hydrogel sponges implanted in the rabbit cornea. Biomaterials 1998; 19: 2255–2267.
Vijayasekaran S, Hicks CR, Chirila TV, Fitton JH, Clayton AB, Lou X et al. Histologic evaluation during healing of hydrogel core-and-skirt keratoprostheses in the rabbit eye. Cornea 1997; 16: 352–359.
Ziegelaar B, Fitton JH, Clayton AB, Platten ST, Steer J, Chirila TV . The modulation of cellular responses to poly(2-hydroxyethyl methacrylate) hydrogel surfaces: phosphorylation decreases macrophage collagenase production in vitro. J Biomater Sci Polym Ed 1998; 9: 849–862.
Lou X, Vijayasekaran S, Chirila TV, Maley MA, Hicks CR, Constable IJ . Synthesis, physical characterization, and biological performance of sequential homointerpenetrating polymer network sponges based on poly(2-hydroxyethyl methacrylate). J Biomed Mater Res 1999; 47: 404–411.
Hicks C, Crawford G, Chirila T, Wiffen S, Vijayasekaran S, Lou X et al. Development and clinical assessment of an artificial cornea. Prog Ret Eye Res 2000; 19: 149–170.
Hicks CR, Werner L, Vijayasekaran S, Mamalis N, Apple DJ . Histology of AlphaCor skirts: evaluation of biointegration. Cornea 2005; 24: 933–940.
Hicks CR, MaeVie O, Crawford GJ, Constable IJ . A risk score as part of an evidence-based approach to the selection of corneal replacement surgery. Cornea 2005; 24: 523–530.
Hicks CR, Crawford GJ . Melting after keratoprosthesis implantation: the effects of medroxyprogesterone. Cornea 2003; 22: 497–500.
Hicks CR, Crawford GJ, Tan DT, Snibson GR, Sutton GL, Gondhowiardjo TD et al. Outcomes of implantation of an artificial cornea. AlphaCor: effects of prior ocular herpes simplex infection. Cornea 2002; 21: 685–690.
Hicks CR, Chirila TV, Werner L, Crawford GJ, Apple DJ, Constable IJ . Deposits in artificial corneas: risk factors and prevention. Clin Experiment Ophthalmol 2004; 32: 185–191.
Chirila TV, Morrison DA, Hicks CR, Gridneva Z, Barry CJ, Vijayasekaran S . In vitro drug-induced spoliation of a keratoprosthetic hydrogel. Cornea 2004; 23: 620–629.
Hicks CR, Hamilton S . Retroprosthetic membranes in AlphaCor patients: risk factors and prevention. Cornea 2005; 24: 692–698.
Crawford GJ, Eguchi H, Hicks CR . Two cases of AlphaCor surgery performed using a small incision technique. Clin Experiment Ophthalmol 2005; 33: 10–15.
Eguchi H, Hicks CR, Crawford GJ, Tan DT, Sutton GR . Cataract surgery with AlphaCor. J Cataract Refract Surg 2004; 30: 1486–1491.
Netland PA, Terada H, Dohlman CH . Glaucoma associated with keratoprosthesis. Ophthalmology 1998; 105: 751–757.
Nouri M, Terada H, Alfonso EH, Foster CS, Durand ML, Dohlman CH . Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol 2001; 119: 484–489.
Coassin M, Zhang C, Gree WR, Aquavella JV, Akpek EK . Histopathologic and immunologic aspects of AlphaCor artificial corneal failure. Am J Ophthalmol 2007; 144: 699–704.
Zerbe BR, Belin MW, Ciolino JB . Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology 2006; 113: 1779–1784.
Bradle JC, Hernandez EG, Schwab IR, Mannis MJ . Boston type 1 keratoprosthesis: The University of California Davis Experience. Cornea 2009; 28: 321–327.
Chew HF, Ayres DB, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JS et al. Boston keratoprostesis outcomes and complications. Cornea 2009; 28: 989–996.
Liu C, Paul B, Tandon R, Lee E, Fong K, Mavrikakis I et al. The Osteo-Odonto-Keratoprosthesis (OOKP). Semin Ophthalmol 2005; 20: 113–128.
Falcinelli G, Falsini B, Taloni M, Colliardo P, Falcinelli G . Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: long term anatomical and functional outcomes in 181 cases. Arch Ophthalmol 2005; 123: 1319–51329.
Tan DTH, Tay ABG, Theng JTS, Lye KW, Parthasarathy A, Por YM et al. Keratoprosthesis surgery for end-stage corneal blindness in Asian eyes. Ophthalmology 2008; 3: 503–510.
Pineda R, Shiuey Y . The keraklear artificial cornea, a novel keratoptosthesis. Tech Ophthalmlol 2009; 7: 101–104.
Acknowledgements
Supported in part by research project MZO 00179906 from the Ministry of Health, Prague, Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jirásková, N., Rozsival, P., Burova, M. et al. AlphaCor artificial cornea: clinical outcome. Eye 25, 1138–1146 (2011). https://doi.org/10.1038/eye.2011.122
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2011.122
Keywords
This article is cited by
-
The first-in-human implantation of the CorNeat keratoprosthesis
Eye (2023)
-
Preparation and In Vitro Characterization of Gelatin Methacrylate for Corneal Tissue Engineering
Tissue Engineering and Regenerative Medicine (2022)
-
Hydrogel membranes based on genipin-cross-linked chitosan blends for corneal epithelium tissue engineering
Journal of Materials Science: Materials in Medicine (2012)