Abstract
Purpose
To compare the effectiveness of intravitreal injection of bevacizumab and ranibizumab in patients with treatment-naïve polypoidal choroidal vasculopathy (PCV).
Methods
A total of 66 and 60 eyes of 121 consecutive patients who received intravitreal bevacizumab (1.25 mg) or ranibizumab (0.5 mg) injection for treatment of PCV were retrospectively reviewed. After initial three loading injections by month, injection was performed as needed. Main outcome measures included best corrected visual acuity (BCVA), foveal center thickness (FCT) as assessed by spectral domain optical coherence tomography (SD-OCT), and change in polypoidal lesion on indocyanine green angiography (ICGA).
Results
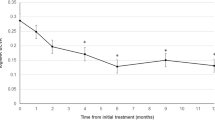
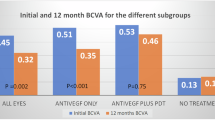
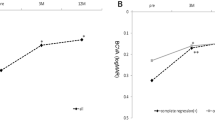
At 12 months, average number of injections was 4.72±1.84 in the bevacizumab group and 5.52±1.54 in the ranibizumab group. Mean logarithm of the minimum angle of resolution of BCVA from baseline at 12 months after injection improved by 0.11 in the bevacizumab group (P=0.02) and by 0.14 in the ranibizumab group (P=0.01). Average FCT decreased from 368±62.48 to 298±40.77 μm in the bevacizumab group (P=0.01) and from 371±50.79 to 286±36.93 μm in the ranibizumab group (P=0.01). Polyp regression rate was 24.2% (16 eyes out of 66 eyes) in the bevacizumab group and 23.3% (14 eyes out of 60 eyes) in the ranibizumab group. There was no statistically significant difference in BCVA improvement achieved, FCT improvement achieved, and polyp regression rate between groups.
Conclusion
Intravitreal injections of bevacizumab and ranibizumab have similar effects in stabilization of visual acuity, macular edema, and regression of polypoidal complex with PCV eyes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B . Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990; 10: 1–8.
Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA . The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 1997; 115: 478–485.
Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 2003; 121: 1392–1396.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA . Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995; 15: 100–110.
Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology 2004; 111: 1576–1584.
Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmololgy 2008; 115: 141–146.
Akaza E, Yuzawa M, Matsumoto Y, Kashiwakura S, Fujita K, Mori R . Role of photodynamic therapy in polypoidal choroidal vasculopathy. Jpn J Ophthalmol 2007; 51: 270–277.
Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 2008; 92: 70–73.
Lai TY, Chan WM, Liu DT, Luk FO, Lam DS . Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol 2008; 92: 661–666.
Cho M, Barbazetto IA, Freund KB . Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol 2009; 148: 70–78.
Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol 2006; 141: 456–462.
Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M . Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol 2004; 88: 809–815.
Kokame GT, Yeung L, Lai JC . Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy (PEARL study): 6-month results. Br J Ophthalmol 2010; 94: 297–301.
CATT research group Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364: 1897–1908.
Holladay JT . Proper method for calculating average visual acuity. J Refract Surg 1997; 13: 388–391.
Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology 2004; 111: 1576–1584.
Gomi F, Tano Y . Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol 2008; 19: 208–212.
Lantry LE . Ranibizumab, a mAb against VEGF-A for the potential treatment of age-related macular degeneration and other ocular complications. Curr Opin Mol Ther 2007; 9: 592–602.
Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina 2006; 26: 262–269.
Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol 1999; 27: 536–544.
Kuroiwa S, Tateiwa H, Hisatomi T, Ishibashi T, Yoshimura N . Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Experiment Ophthalmol 2004; 32: 297–302.
Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N . Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol 2002; 86: 1093–1098.
Lai TY . Verteporfin PDT and ranibizumab combination therapy for symptomatic macular polypoidal choroidal vasculopathy: EVEREST result. Invest Ophthalmol Vis Sci 2010; 51: E-abstract 2228.
Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, Mandai M et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina 2007; 27: 335–341.
Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M . Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol 2004; 88: 809–815.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cho, H., Kim, J., Lee, D. et al. Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye 26, 426–433 (2012). https://doi.org/10.1038/eye.2011.324
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2011.324
Keywords
This article is cited by
-
Idiopathic polypoidal choroidal vasculopathy: a review of literature with clinical update on current management practices
International Ophthalmology (2021)
-
Intravitreal aflibercept for active polypoidal choroidal vasculopathy without active polyps
Scientific Reports (2019)
-
Clinical outcomes in Caucasian patients with polypoidal choroidal vasculopathy
Eye (2018)
-
Submacular hemorrhage and grape-like polyp clusters: factors associated with reactivation of the lesion in polypoidal choroidal vasculopathy
Eye (2017)
-
Anti-vascular endothelial growth factor monotherapy for polypoidal choroidal vasculopathy with polyps resembling grape clusters
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)