Abstract
Purpose
To determine whether a Pro Re Nata (PRN) regimen with three initial mandatory loading doses results in better functional and anatomical outcome compared with a PRN regimen without initial loading when using intravitreal bevacizumab in patients with minimal classic or occult choroidal neovascularisation secondary to age-related macular degeneration.
Methods
Patients were randomised (1 : 1) to Loading (LD group) or No Loading (NLD group) and treated with open label intravitreal bevacizumab. In the LD group, patients received two mandatory doses after the baseline dose before entering the PRN phase and in the NLD group, patients did not receive mandatory doses after the baseline dose. Six-weekly evaluations were performed up to week 54 and retreatment was done based on OCT criteria. Visual stability and reduction in central retinal thickness were compared between groups.
Results
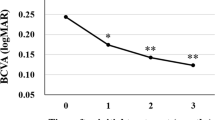
49 patients were in the NLD group and 50 patients were in the LD group. At the 12-month end point, 84% of the patients in the LD group achieved visual stability (<15 letter loss) compared with 67% of the patients in the NLD group (P<0.05). The mean reduction in central macular thickness was 105.35 μm in the LD group and 81.45 μm in the NLD group (P>0.05). There was no significant difference in scores of VFQ-25 questionnaire testing between the two groups and no serious ocular or systemic side effects were observed.
Conclusion
The results supported our hypothesis that a loading dose leads to slightly better visual stability in terms of proportions of patients experiencing moderate visual loss, but did not support the hypothesised difference in anatomical outcome.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012; 119: 1399–1411.
CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364: 1897–1908.
Arias L, Caminal JM, Casas L, Masuet C, Badia MB, Rubio M et al. A study comparing two protocols of treatment with intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Br J Ophthalmol 2008; 92: 1636–1641.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE et al. Comparison of age-related macular degeneration treatments trials (catt) research group, ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two year results. Ophthalmology 2012; 119: 1388–1398.
Gupta B, Adewoyin T, Patel SK, Sivaprasad S . Comparison of two intravitreal ranibizumab treatment schedules for neovascular age-related macular degeneration. Br J Ophthalmol 2011; 95 (3): 386–390.
El-Mollayess G, Mahfoud Z, Schakal A, Salti H, Jaafar D, Bashshur ZF . Fixed-interval versus OCT-guided variable dosing of intravitreal bevacizumab in the management of neovascular age-related macular degeneration: A 12-month randomized prospective study. Am J Ophthalmol 2012; 153: 481–489.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et alANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1432–1444.
Singer MA, Awh CC, Sadda S, Freeman WRAntoszyk AN, Wong P, Tuomi L . HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology 2012; 119: 1175–1183.
Olajumoke S, Gupta B, Vemala R, Sivaprasad S . Visual acuity outcomes in ranibizumab-treated neovascular age-related macular degeneration; stratified by baseline vision. Clin Exp Ophthalmol 2011; 39: 3–5.
Williams TA, Blyth CP . Outcome of ranibizumab treatment in neovascular age related macular degeneration. Eye 2011; 25: 1617–1621.
Rathore D, Oyede T, Narendran N, Yang YC . Snellen versus logMAR visual acuity charts for evaluating driving standards in patients with neovascular macular degeneration. Br J Vis Impair 2012; 30: 160–167.
Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, Axer-Siegel R et alEXCITE Study Group. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 2011; 118 (5): 831–839.
Tufail A, Patel PJ, Egan C, Hykin P, da Cruz L, Gregor Z et alABC Trial Investigators. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ 2010; 340: c2459.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007; 143 (4): 566–583.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD et al. VIEW 1 and VIEW 2 study groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012; 119: 2537–2548.
Suner IJ, Yau L, Lai P . HARBOR study: one-year results of efficacy and safety of 2.0 mg versus 0.5 mg ranibizumab in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Paper presented at The Association for Research in Vision and Ophthalmology Annual Meeting, 1 May 2011, Fort Lauderdale, FL. (abstract no. 3677).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Menon, G., Chandran, M., Sivaprasad, S. et al. Is it necessary to use three mandatory loading doses when commencing therapy for neovascular age-related macular degeneration using bevacizumab? (BeMOc Trial). Eye 27, 959–963 (2013). https://doi.org/10.1038/eye.2013.93
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2013.93
Keywords
This article is cited by
-
Aflibercept in Diabetic Macular Oedema Previously Refractory to Standard Intravitreal Therapy: An Irish Retrospective Study
Ophthalmology and Therapy (2018)
-
Intravitreal Bevacizumab and Cardiovascular Risk in Patients with Age-Related Macular Degeneration: Systematic Review and Meta-Analysis of Randomized Controlled Trials and Observational Studies
Drug Safety (2016)