Summary
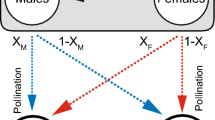
A model is presented which expresses the seed production of dioecious and gynodioecious animal-pollinated Angiosperms in terms of the relative seed-fecundity of the sexes, the number of pollinator visits to each flower and the sex ratio. The model predicts that the maximum seed set occurs when females predominate, providing the pollinators visit each flower more than once and the seed set of males is not high.
There is strong evidence that a marked preponderance of females in species of four genera is due to more frequent fertilisation by female-determining pollen nuclei than by male-determining nuclei. Two hypotheses have been proposed to explain this differential fertilisation.
Several objections are raised to the hypothesis of Lewis (1942), Mulcahy (1967) and Kaplan (1972) that female-predominant sex ratios have been selected because they maximise the total seed production of populations. It is considered that the hypothesis of Smith (1963) that the differential fertilisation is a consequence of the genetic differentiation of sex chromosomes offers a more likely explanation of female-predominant sex ratios.
Similar content being viewed by others
Article PDF
References
Allen, C E. 1940. The genotypic basis of sex-expression in Angiosperms. Bot Rev, 6. 227–300.
Burrows, C J. 1960. Studies in Pimelea. I. The breeding system. Trans Roy Soc New Zealand, 88, 29–45.
Connor, H E. 1965. Breeding systems in New Zealand grasses. V. Naturalised species of Cortaderia. New Zealand J Bot, 3, 17–23.
Correns, C. 1928. Bestimmung, Vererbung und Verteilung des Geschlechtes bei den hoheren Pflanzen. Handb Vererbungsw, 2, 1–138.
Dronamraju, K R. 1965. The function of the Y chromosome in man, animals and plants. Adv Genet, 13, 227–310.
Elkington, T T, and Woodell, S R J. 1963. Potentilla fruticosa L. (Dasiphora fruticosa (L.) Rydb.). J Ecology, 51, 769–781.
Fisher, R A. 1930. The Genetical Theory of Natural Selection. Oxford University Press, Oxford.
Godley, E J. 1964. Breeding systems in New Zealand plants. 3. Sex ratios in some natural populations. New Zealand J Bot, 2, 205–212.
Grewal, M S, and Ellis, J R. 1972. Sex determination in Potentilla fruticosa. Heredity, 29, 359–362.
Harris, W. 1968. Environmental effects on the sex ratio of Rumex acetosella L. Proc New Zealand Ecol Soc, 15, 51–54.
Kaplan, S M. 1972. Seed production and sex ratio in anemophilous plants. Heredity, 28, 281–285.
Kihara, H, and Hirayoshi, I. 1932. Die Geschlechtschromosomen von Humulus japonicus Sieb. et Zucc. 8th Congr Jap Ass Adv Sci, 363–367.
Lawrence, C W. 1963. Genetic studies on wild populations of Melandrium. II. Flowering time and plant weight. Heredity, 18, 149–163.
Lewis, D. 1942. The evolution of sex in flowering plants. Cambr Phil Soc Biol Rev, 17, 46–67.
Löve, A. 1943. Cytogenetic studies on Rumex subgenus Acetosella. Hereditas, 30, 1–136.
Lloyd, D G. 1973a. Sex ratios in sexually dimorphic Umbelliferae. Heredity (in press).
Lloyd, D G. 1973b. Theoretical sex ratios of dioecious and gynodioecious Angiosperms. Heredity, 31, 11–34.
Mulgahy, D L. 1967. Optimal sex ratio in Silene alba. Heredity, 22, 411–423.
Mulcahy, D L. 1971. A correlation between gametophytic and sporophytic haracteristics in Zea mays L. Science, 171, 1155–1156.
Nei, M. 1970. Accumulation of non-functional genes on sheltered chromosomes. Amer Nat, 104, 311–322.
Putwain, P D, and Harper, J L. 1972. Studies in the dynamics of plant populations. V. Mechanisms governing the sex ratio in Rumex acetosa and R. acetosella. J Ecol, 60, 113–129.
Riede, W. 1925. Beitrage zum Geschlechts-und Anpassungs-problem. Flora, 18/19, 421–452.
Ross, M D. 1969. Digenic inheritance of male sterility in Plantago lanceolata. Can J Genet Cytol, 11, 739–744.
Shaw, R F, and Mohler, J D. 1953. The selective significance of the sex ratio. Amer Nat, 87, 337–342.
Singh, R B, and Smith, B W. 1971. The mechanism of sex determination in Rumex acetosella. Theor Appl Genetics, 41, 360–364.
Smith, B W. 1963. The mechanism of sex determination in Rumex hastatulus. Genetics, 48, 1265–1288.
Valdeyron, G. Assouad, W, and Dommée, B. 1970. Coéxistence des determinismes génique et cytoplasmique de la stérilité mâle: recherche d'une hypothèse explicative. In Symposium Stérilité Mâle en Horticulture, Versailles.
Westergaard, M. 1958. The mechanism of sex determination in dioecious flowering plants. Adv Genet, 5, 217–281.
Williams, W. 1964. Genetical principles and plant breeding. Blackwell, Oxford.
Żuk, J. 1963. An investigation on polyploidy and sex determination within the genus Rumex. Acta Soc Bot Polon, 32, 5–67.
Żuk, J. 1970. Function of Y chromosomes in Rumex thyrsiflorus. Theor Appl Genetics, 40, 124–129.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lloyd, D. Female-predominant sex ratios in angiosperms. Heredity 32, 35–44 (1974). https://doi.org/10.1038/hdy.1974.3
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1974.3
This article is cited by
-
The influence of environmental conditions on sex ratio in a dioecious plant Pistacia vera L
Plant Physiology Reports (2022)
-
Plant sex determination and sex chromosomes
Heredity (2002)
-
The effect of drought stress on the sex ratio variation ofSilene otites
Folia Geobotanica (2000)
-
Insects and plants in the pollination ecology of the boreal zone
Ecological Research (1993)
-
The sex ratlo in a dioecious endemic plant, Silene dielinis
Genetica (1984)