Abstract
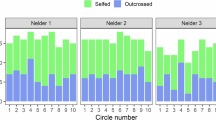
Inbreeding depression is likely to be a common selective force opposing the automatic selective advantage of self-fertilization in self-compatible plants and animals, yet relatively few studies have measured both the breeding system and inbreeding depression in natural populations. In this study, I estimated the frequency of selfing, using data obtained by gel electrophoresis, in two annual populations of the monkeyflower Mimulus guttatus for 2 years, and measured the relative performance of selfed and outcrossed progeny over several stages of the life cycle in both the field and the greenhouse. Rates of outcrossing were not significantly different from 1.0 in either population in 1989, but both populations exhibited significant and moderate amounts of selfing in 1990. Outcrossing rates were significantly different between years for the Cone Peak population but not for the Iron Mountain population. Significant inbreeding depression was detected for almost every component of fitness measured, including germination success, survival to flowering, and flower, fruit and seed production in the native field environments for both populations. The final cumulative value of inbreeding depression, calculated as one minus the relative total seed production of selfed to outcrossed progeny, was 0.69 for Iron Mountain and 0.64 for Cone Peak. Inbreeding depression was also severe in the greenhouse experiments, even though fitness components only up to flower production were measured: 0.52 for Iron Mountain and 0.48 for Cone Peak. These results are consistent with theoretical predictions of the magnitude of inbreeding depression in primarily outcrossing populations, and indicate that inbreeding depression is an important factor in the maintenance of outcrossing in these populations.
Similar content being viewed by others
Article PDF
References
Aide, T M. 1986. The influence of wind and animal pollination on variation in outcrossing rates. Evolution, 40, 434–435.
Antonovics, J. 1968. Evolution in closely adjacent plant populations. V. Evolution of self-fertility. Heredity, 23, 219–238.
Baker, H G. 1955. Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution, 11, 449–460.
Barrett, S C H, and Eckert, C G. 1990. Variation and evolution of mating systems in seed plants. In: S. Kawano (ed.) Biological Approaches and Evolutionary Trends in Plants. Academic Press, San Diego, CA, pp. 229–254.
Campbell, R B. 1986. The interdependence of mating structure and inbreeding depression. Theoret Pop Biol, 30, 232–244.
Charlesworth, B. 1980. The cost of sex in relation to mating system. J Theoret Biol, 84, 655–671.
Charlesworth, B, Charlesworth, D, and Morgan, M T. 1990. Genetic loads and estimates of mutation rates in highly inbred plant populations. Nature, 347, 380–382.
Charlesworth, B, Morgan, M T, and Charlesworth, D. 1991. Multilocus models of inbreeding depression with synergistic selection and partial self-fertilization. Genet Res, Camb, 57, 177–194.
Charlesworth, D, and Charlesworth, B. 1979. The evolutionary genetics of sexual systems in flowering plants. Proc Roy Soc Lond B, 205, 513–530.
Charlesworth, D, and Charlesworth, B. 1987. Inbreeding depression and its evolutionary consequences. Ann Rev Ecol Syst, 18, 237–268.
Charlesworth, D, and Charlesworth, B. 1990. Inbreeding depression with heterozygote advantage and its effect on selection for modifiers changing the outcrossing rate. Evolution, 44, 870–888.
Charlesworth, D, Morgan, M T, and Charlesworth, B. 1990. Inbreeding depression, genetic load and the evolution of outcrossing rates in a multi-locus system with no linkage. Evolution, 44, 1469–1489.
Clayton, J W, and Tretiak, D N. 1972. Amine-citrate buffers for pH control in starch gel electrophoresis. J Fisheries Res Board Can, 29, 1169–1172.
Crow, J F, and Simmons, M J. 1983. The mutation load in Drosophila. In: Ashburner, M., Carson, H. L. and Thompson, J. N. (eds.) The Genetics and Biology of Drosophila Academic Press, London, pp. 1–35.
Darwin, C R. 1862. The Various Contrivances by which Orchids are Fertilized by Insects. John Murray, London.
Darwin, C R. 1877. The Different Forms of Flowers on Plants of the Same Species. John Murray, London.
Dole, J A. 1990. Role of corolla abscission in delayed self-pollination of Mimulus guttatus (Scrophulariaceae). Amer J Bot, 77, 1505–1507.
Dole, J A, and Ritland, K. 1993. Inbreeding depression in two Mimulus taxa measured by multigenerational changes in the inbreeding coefficient. Evolution, 47, 361–373.
Dudash, M R. 1990. Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae): a comparison in three environments. Evolution, 44, 1129–1139.
Feldman, M W, and Christiansen, F B. 1984. Population genetic theory of the cost of inbreeding. Amer Nat, 123, 642–653.
Fisher, R A. 1941. Average excess and average effect of a gene substitution. Ann Eugen, 11, 53–63.
Harris, J E. 1979. The pollination ecology and sexual reproductive ecology of Mimulus guttatus along an elevational gradient Master's Thesis, University of Utah, Salt Lake City.
Holsinger, K E. 1988. Inbreeding depression doesn't matter: The genetic basis of mating system evolution. Evolution, 42, 1235–1244.
Holsinger, K E, and Feldman, M W, and Christiansen, F B. 1984. The evolution of self-fertilization in plants: a population genetic model. Amer Nat, 124, 446–453.
Holtsford, T P, and Ellstrand, N C. 1990. Inbreeding effects in Clarkia tembloriensis (Onagraceae) populations with different natural outcrossing rates. Evolution, 44, 2031–2046.
Jain, S K. 1978. Breeding system in Limnanthes alba: several alternative measures. Amer J Bot, 65, 272–275.
Johnston, M O. 1992. Effects of cross and self-fertilization on progeny fitness in Lobelia cardinalis and L. siphilitica. Evolution, 46, 688–702.
Karron, J D. 1989. Breeding systems and levels of inbreeding depression in geographically restricted and widespread species of Astragalus (Fabaceae). Amer J Bot, 76, 331–340.
Kohn, J. 1988. Why be female? Nature, 335, 431–433.
Lande, R, and Schemske, D W. 1985. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution, 39, 24–40.
Levin, D A. 1989. Inbreeding depression in partially self-fertilizing Phlox. Evolution, 43, 1417–1423.
Levin, D A. 1991. The effect of inbreeding on seed survivorship in Phlox. Evolution, 45, 1047–1049.
Lloyd, D G. 1979. Some reproductive factors affecting the selection of self-fertilization in plants. Amer Nat, 113, 67–79.
Lloyd, D G. 1980. Demographic factors and mating patterns in angiosperms. In: Solbrig, O. T (ed.) Demography and Evolution in Plant Populations Blackwell, Oxford, pp. 67–88.
Lynch, M. 1988. Design and analysis of experiments on random genetic drift and inbreeding depression. Genetics, 120, 791–807.
Macnair, M R, and Cumbes, Q J. 1989. The genetic architecture of interspecific variation in Mimulus. Genetics, 122, 211–222.
Macnair, M R, Macnair, V E, and Martin, B E. 1989. Adaptive speciation in Mimulus: an ecological comparison of M. cupriphilus with its presumed progenitor, M. guttatus. New Phytol, 112, 269–279.
Manasse, R S, and Pinney, K. 1991. Limits to reproductive success in a partially self-incompatible herb: fecundity depression at serial life-cycle stages. Evolution, 45, 712–720.
Maynard Smith, J. 1977. The sex habit in plants and animals. In: Christiansen, F. B. and Fenchel, T. M. (eds) Measuring Selection in Natural Populations Springer-Verlag, Berlin, pp. 265–273.
Maynard Smith, J. 1978. The Evolution of Sex. Cambridge University Press, Cambridge.
McClure, S. 1973. Allozyme variability in natural populations of the yellow monkey-flower, Mimulus guttatus, located in the north Yuba River drainage. Ph. D. thesis, University of California, Berkeley.
Price, M V, and Waser, N M. 1979. Pollen dispersal and optimal outcrossing in Delphinium nelsoni. Nature, 211, 294–297.
Ritland, C, and Ritland, K. 1989. Variation of sex allocation among eight taxa of the Mimulus guttatus species complex. Amer J Bot, 76, 1731–1739.
Ritland, K. 1990a. Inferences about inbreeding depression based upon changes of the inbreeding coefficient. Evolution, 44, 1230–1241.
Ritland, K. 1990b. A series of FORTRAN computer programs for estimating plant mating systems. J Heredity, 81, 235–237.
Ritland, K, and Ganders, F R. 1987a. Covariation of selfing rates with parental gene fixation indices within populations of Mimulus guttatus. Evolution, 41, 760–771.
Ritland, K, and Ganders, F. 1987b. Crossability of Mimulus guttatus in relation to components of gene fixation. Evolution, 41, 772–786.
Ritland, K, and Jain, S K. 1981. A model for the estimation of outcrossing rate and gene frequencies using n independent loci. Heredity, 47, 35–52.
Schemske, D W. 1978. Evolution of reproductive characters in Impatiens (Balsaminaceae): the significance of cleistogamy and chasmogamy. Ecology, 59, 596–613.
Schemske, D W. 1983. Breeding system and habitat effects on fitness in three neotropical Costus (Zingiberaceae). Evolutional, 523–539.
Schemske, D W. 1984. Population structure and local selection in Impatiens pallida (Balsaminaceae), a selfing annual. Evolution, 38, 817–832.
Schemske, D W, and Pautler, L P. 1984. The effects of pollen composition on fitness components in a neotropical herb. Oecologia (Berlin), 62, 31–36.
Schemske, D W, and Lande, R. 1985. The evolution of self-fertilization and inbreeding depression in plants. II. Empirical observations. Evolution, 39, 41–52.
Schmitt, J, and Ehrhardt, D W. 1990. Enhancement of inbreeding depression by dominance and suppression in Impatiens capensis. Evolution, 44, 269–278.
Schmitt, J, and Gamble, S E. 1990. The effect of distance from the parental site on offspring performance in Impatiens capensis: a test of the local adaptation hypothesis. Evolution, 44, 2022–2030.
Schoen, D J. 1983. Relative fitnesses of selfed and outcrossed progeny in Gilia achilleifolia (Polemoniaceae). Evolution, 37, 292–301.
Selander, R K, Smith, M H, Yang, S Y, Johnson, W E, and Gentry, J B. 1971. Biochemical polymorphism and systematics in the genus Peromyscus I Variations in the old-field mouse (P polionotus). University of Texas Publishers, 103, 49–90.
Simmons, M J, and Crow, J F. 1977. Mutations affecting fitness in Drosophila populations. Ann Rev Genet, 11, 49–78.
Sokal, R R, and Rohlf, F J. 1981. Biometry, 2nd ed. Freeman, New York.
Soltis, D E, and Soltis, P S. 1992. The distribution of selfing rates in homosporous ferns. Amer J Bot, 79, 97–100.
Sprague, G F. 1983. Heterosis in maize: theory and practice. In: Frankel, R. (ed.) Heterosis: Reappraisal of Theory and Practice Springer-Verlag, Berlin, pp. 48–70.
Stebbins, G L. 1990. Variation and Evolution in Plants. Columbia University Press, New York.
Stebbins, G L. 1957. Self-fertilization and population variation in the higher plants. Amer Nat, 91, 337–354.
Stevens, J P, and Bougourd, S M. 1988. Inbreeding depression and the outcrossing rate in natural populations of Allium schoenoprasum L. (wild chives). Heredity, 60, 257–261.
Waller, D M. 1979. The relative costs of selfed and out-crossed seeds in Impatiens capensis (Balsaminaceae). Amer J Bot, 66, 313–320.
Waller, D M. 1986. IS there disruptive selection for self fertilization? Amer Nat, 128, 421–426.
Weller, S G, and Ornduff, R. 1991. Pollen tube growth and inbreeding depression in Amsinckia grandiflora (Bora-ginaceae). Amer J Bot, 78, 801–804.
Willis, J H. 1991. The role of inbreeding depression in the evolution of two partially self-fertilizing populations of Mimulus guttatus Ph. D. thesis, University of Chicago, IL.
Willis, J H. 1992. Genetic analysis of inbreeding depression due to chlorophyll-deficient lethals in Mimulus guttatus. Heredity, 69, 562–572.
Willis, J H. 1993. Effects of different levels of inbreeding on fitness components in Mimulus guttatus. Evolution, in press.
Ziehe, M, and Roberds, J H. 1989. Inbreeding depression due to overdominance in partially self-fertilizing plant populations. Genetics, 121, 861–868.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Willis, J. Partial self-fertilization and inbreeding depression in two populations of Mimulus guttatus. Heredity 71, 145–154 (1993). https://doi.org/10.1038/hdy.1993.118
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1993.118
Keywords
This article is cited by
-
Stresses affect inbreeding depression in complex ways: disentangling stress-specific genetic effects from effects of initial size in plants
Heredity (2021)
-
Interactive effects between donor and recipient species mediate fitness costs of heterospecific pollen receipt in a co-flowering community
Oecologia (2019)
-
Examining the genetic integrity of a rare endemic Colorado cactus (Sclerocactus glaucus) in the face of hybridization threats from a close and widespread congener (Sclerocactus parviflorus)
Conservation Genetics (2015)
-
Effects of herkogamy and inbreeding on the mating system of Mimulus luteus in the absence of pollinators
Evolutionary Ecology (2010)
-
Genetic variation and constraints on the evolution of defense against spittlebug (Philaenus spumarius) herbivory in Mimulus guttatus
Heredity (2009)