Abstract
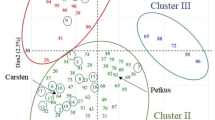
Isoenzymes were used to examine genetic variability within and among populations of chestnut (Castanea sativa Mill.) and wild cherry (Prunus avium L.). Isoenzymes were sufficiently robust to allow the attribution of chestnut shoots to stumps; other markers were required to confirm the estimations of wild cherry clone sizes. No genetic structure was observed within populations of the two species and no significant departures from Hardy-Weinberg equilibrium were detected for the isoenzymes tested excluding one locus. F-statistics and measurements of genetic distances revealed little genetic differentiation among populations of these species. This could be explained by the influence of human activities and the limited number of generations observed for these species since the last glaciation.
Similar content being viewed by others
Article PDF
References
Bonnefoi, C. 1984. Etude du polymorphisme enzymatique des populations forestières de châtaignier (Castanea saliva Mill.). Thesis, USTL Montpellier, France.
Bousquet, J, Cheliak, W M, and Lalonde, M. 1987. Allozyme variability in natural population of green alder (Alnus crispa) in Quebec. Genome, 29, 345–352.
Cheliak, W M, and Dancik, B P. 1982. Genie diversity of natural populations of a clone forming tree Populus tremuloides. Can J Genet Cytol, 24, 611–616.
Clapper, R B. 1954. Chestnut breeding, techniques and results. J Hered, 45, 106–114, 201–208.
Crane, B. 1923. Report on tests of self-sterility and cross-incompatibility in plums, cherries and apples at the John Innes Horticultural Institution II. J Pomol Hon Science, 3, 67–84.
Cuguen, J, Merzeau, D, and Thiebaut, B. 1989. Genetic structure of the European beech stands Fagus silvatica L. F- statistics and importance of mating system characteristics in their evolution. Heredity, 60, 91–100.
Fineschi, S, Gillet, E, and Malvolti, M E. 1990. Genetics of sweet chestnut (Castanea sativa Mill).II. Genetic analysis of zymograms of single tree offspring. Silvae Genetica, 39, 188–193.
Frascaria, N, and Lefranc, M. 1992. Le commerce de la châtaigne: un nouvel aspect dans l'étude de la différencia-tion génétique de populations de châtaigniers (Castanea sativa Mill.) en France. Ann Sei Forest, 1, 75–79.
Hamrick, J L. 1989. Isozymes and the analysis of genetic structure in plant population. In: Soltis, D. E. and Soltis, P. S. (eds). Isozymes Plant Biology. Discories Press, Portland, pp. 87–105.
Harris, H, and Hopkinson, D A. 1976. Handbook of Enzyme Electrophoresis in Human Genetics. North Holland Publishing Company Inc., New York.
Hiebert, R D, and Hamrick, J L. 1982. Patterns and levels of genetic variation in great basin bristlecone pine (Pinus longaeva Bailey). Evolution, 37, 302–310.
Kremer, A, Petit, P, Zanetto, A, Fougere, V, Ducousso, A, Wagner, D, and Chauvin, C. 1991. Nuclear and organelle gene diversity in Q. robur and Q. petraea. In: Muller-Starck, G. and Ziette, M. (eds) Genetic Variations in European Forest trees, Saverländer Verlag, 141–166.
Lewis, D, and Crowe, L K. 1954. Study of the incompatibility gene. IV types of mutations in Prunus avium L. Heredity, 8 (3), 357–363.
Loveless, M D, and Hamrick, J L. 1984. Ecological determinants of genetic structure in plant populations. Ann Rev Syst, 15, 65–95.
Meinzel, S, and Market, C L. 1967. Malate dehydrogenase isozymes of the marine snail, Ilyanassa obsoleta. Arch Biochem Biophys, 122, 753–765.
Nei, M. 1972. Genetic distance between populations. Am Nat, 106, 283–292.
Nei, M. 1973. Analysis of gene diversity in subdivided populations. Proc Nat Acad Sci USA, 70, 3321–3323.
Nei, M. 1977. F-statistics and analysis of gene diversity in subdivided populations. Am Hum Genet, 41, 225–233.
Nei, M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89, 583–590.
Nei, M. 1986. Definition and estimation of fixation indices. Evolution, 40, 643–645.
Pigliucci, S, Benedetelli, S, and Villani, F. 1990. Spatial patterns of genetic variability in Italian chestnut (Castanea sativa Mill.). Can J Bot, 9, 1962–1967.
Santi, F, and Lemoine, M. 1991. Genetic markers for Prunus avium L.: inheritance and linkage of isozyme loci. Ann Soc For, 47, 131–139.
Tanksley, S D, and Rick, C M. 1980. Isozyme gene linkage map of the tomato: applications in genetics and breeding. TheorAppl Genet, 57, 161–170.
Valizadeh, M. 1978. Aspects génétiques et agronomiques de l'étude de la variabilité des protéines chez les plantes supérieures Cas du Ficus carita L. Thesis, USTL Montpellier, France.
Villani, F, Malvolti, M E, Fineschi, S, Bimbi, R, and Paciucci, M. 1986. Electrophoretic variations in Castanea sativa M. from Italy. 5th International Congress on Isozymes. Island of Kos, Greece, May 26–29.
Wright, S. 1951. The genetical structure of populations. Ann Eugen, 15, 323–354.
Wright, S. 1965. The interpretation of population structure by F-statistics with special regard to system of mating. Evolution, 19, 395–420.
Yacine, A, and Lumaret, R. 1988. Distribution spaciale des génotypes dans une population de chênes verts (Quercus ilex L.), flux génique et régime de reproduction. Genet Sci Evol, 20, 181–198.
Acknowledgements
We would like to thank Catherine Bourgeois (IDF, Toulouse, France) for access to the different stands, Madeleine Lefranc (University of Paris XI, Orsay, France) for her valuable technical assistance in carrying out electrophoretic analysis and Peng Li and Paul de la Bastide (University of Laval, St-Foy, Canada) for reviewing the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Frascaria, N., Santi, F. & Gouyon, P. Genetic differentiation within and among populations of chestnut (Castanea sativa Mill.) and wild cherry (Prunus avium L.). Heredity 70, 634–641 (1993). https://doi.org/10.1038/hdy.1993.91
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1993.91
Keywords
This article is cited by
-
Different evolutionary processes in shaping the genetic composition of Dendrobium nobile in southwest China
Genetica (2015)
-
Is the genetic diversity of small scattered forest tree populations at the southern limits of their range more prone to stochastic events? A wild cherry case study by microsatellite-based markers
Tree Genetics & Genomes (2011)
-
Population structure and genetic bottleneck in sweet cherry estimated with SSRs and the gametophytic self-incompatibility locus
BMC Genetics (2010)
-
Genetic structure of Cerasus jamasakura, a Japanese flowering cherry, revealed by nuclear SSRs: implications for conservation
Journal of Plant Research (2009)
-
Distribution and fine-scale spatial-genetic structure in British wild cherry (Prunus avium L.)
Heredity (2007)