Abstract
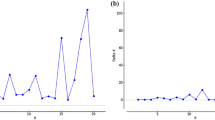
The effect of genetic architecture (linkage relationships, dominance and two forms of non-allelic interaction) on the power of marker studies to detect, locate and analyse the contributions of specific quantitative trait loci (QTLs) to continuous human traits is considered for randomly mating populations in linkage equilibrium under a two-locus model. The expected regression of the within-sibling-pair mean-square on number of alleles identical by descent (IBD) at two marker loci is explored for every possible pair of markers over a region of the genome containing two QTLs linked loosely (50 CM) or more tightly (20 CM).
For the cases examined, it is shown that epistasis between the pair of QTLs reduces considerably the total amount of information available for the location and analysis of the QTL effects. The overall effects of epistasis are more marked when there are duplicate gene interactions (i.e. genes operate in parallel) than when there are complementary interactions (i.e. genes operate in series). However, when there are complementary interactions, the regression approach is almost certain to fail to detect any evidence of epistasis. The numerical analysis suggests that methods of QTL analysis based on IBD in humans are unlikely to offer the resolving power that is desirable if QTLs are to be located precisely unless inheritance is very simple or prohibitively large numbers of highly selected individuals are available.
Similar content being viewed by others
Article PDF
References
Carey, G, and Williamson, J. 1991. Linkage analysis of quantitative traits: increased power by using selected samples. Am J Hum Genet, 49, 786–796.
Caten, C E. 1979. Quantitative genetic variation in fungi. In: Thompson, J. N., Jr and Thoday, J. M. (eds) Quantitative Genetic Variation, pp. 35–60. Academic Press, New York.
Eaves, L J. 1988. Dominance alone is not enough. Behav Genet, 18, 27–33.
Greenberg, D A. 1993. Linkage analysis of ‘necessary’ disease loci versus ‘susceptibility’ loci. Am J Hum Genet, 52, 135–143.
Haley, C S, and Knott, S A. 1992. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity, 69, 315–324.
Haseman, J K, and Elston, R C. 1972. The investigation of linkage between a quantitative trait and a marker locus. Behav Genet, 2, 3–19.
Jinks, J L. 1977. Discussion of ‘Inferring causes of human variation’. J R Stat Soc Lond (A), 140, 353.
Jinks, J L, and Towey, P M. 1976. Estimating the number of genes in a polygenic system by genotype assay. Heredity, 37, 69–81.
Knott, S A, and Haley, C S. 1992. Maximum likelihood mapping of quantitative trait loci using full-sib families. Genetics, 132, 1211–1222.
Lalouel, J M, Rao, D C, Morton, N E, and Elston, R C. 1983. A unified model for complex segregation analysis. Am J Hum Genet, 35, 816–826.
Lander, E S, and Botstein, D. 1986. Strategies for studying heterogeneous genetic traits in humans by using a linkage map of restriction fragment length polymorphisms. Proc Nat Acad Sci USA, 83, 7353–7357.
Lander, E S, and Botstein, D. 1989. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics, 121, 185–199.
Luo, Z W, and Kearsey, M J. 1989. Maximum likelihood estimation of linkage between a marker gene and a quantitative locus. Heredity, 63, 401–408.
Luo, Z W, and Kearsey, M J. 1991. Maximum likelihood estimation of linkage between a marker gene and a quantitative locus. II. Application to backcross and doubled haploid populations. Heredity, 66, 117–124.
Maclean, C J, Morton, N E, and Lew, R. 1975. Analysis of family resemblance. IV. Operational characteristics of segregation analysis. Am J Hum Genet, 27, 365–384.
McGuire, T R. 1992. A biometrical genetic approach to chromosome analysis of Drosophila: detection of epistatic interactions in geotaxis. Behav Genet, 22, 453–467.
Martin, N G, Eaves, L J, Kearsey, M J, and Davies, P. 1978. The power of the classical twin study. Heredity, 9, 539–552.
Mather, K. 1949. Biometrical Genetics, 1st edn. Methuen, London.
Mather, K. 1974. Non-allelic interaction in continuous variation of randomly breeding populations. Heredity, 32, 414–419.
Mather, K, and Jinks, J L. 1982. Biometrical Genetics: The Study of Continuous Variation, 3rd edn. Chapman and Hall, London.
Morton, N E, and Maclean, C J. 1974. Analysis of family resemblance. III. Complex segregation of quantitative traits. Am J Hum Genet, 26, 489–503.
Motro, U, and Thomson, G. 1985. The affected sib pair method. I. Statistical features of the affected sib pair method. Genetics, 110, 525–538.
Nelder, J A, and Wedderburn, R W M. 1972. Generalized linear models. J R Stat Soc Lond (A), 135, 370–384.
Neuman, R J, and Rice, J P. 1992. Two locus models of disease. Genet Epidemiol, 9, 347–365.
NUMERICAL Algorithms Group. 1990. NAG FORTRAN Library: Mark 14. Numerical Algorithms Group, Oxford.
Paterson, A H, Damon, S, Hewitt, J D, Zamir, D, Rabinowitch, H, Lincoln, S E, Lander, E S, and Tanksley, S D. 1991. Mendelian factors underlying quantitative triats in tomato: comparison across species, generations and environments. Genetics, 127, 181–197.
Paterson, A H, Deverna, J W, Lanini, B, and Tanksley, S D. 1990. Fine mapping of quantitative trait loci using selected overlapping recombinant chromosomes in an interspecies cross of tomato. Genetics, 124, 735–742.
Paterson, A H, Lander, E S, Hewitt, J D, Peterson, S, Lincoln, S E, and Tanksley, S D. 1988. Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature, 335, 721–725.
Perkins, J M, and Jinks, J L. 1970. Detection and estimation of genotype-environmental, linkage and epistatic components of variation for a metrical trait. Heredity, 26, 203–209.
Risch, N. 1990. Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet, 46, 222–228.
Schlager, O, and Chao, C S. 1991. The role of dominance and epistasis in the genetic control of blood pressure in rodent models of hypertension. Clin Exp Hypertens, 13, 947–953.
Spickett, S G, and Thoday, J M. 1966. Regular responses to selection: 3. Interaction between located polygenes. Genet Res, 7, 96–121.
Spielman, R S, McGinnis, R E, and Ewens, W J. 1993. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet, 52, 506–516.
Van Der Veen, J H. 1959. Tests of non-allelic interaction and linkage for quantitative characters in generations derived from two diploid pure lines. Genetica, 30, 201–232.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eaves, L. Effect of genetic architecture on the power of human linkage studies to resolve the contribution of quantitative trait loci. Heredity 72, 175–192 (1994). https://doi.org/10.1038/hdy.1994.25
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1994.25
Keywords
This article is cited by
-
Epistasis: too often neglected in complex trait studies?
Nature Reviews Genetics (2004)
-
Genome-wide scans of three independent sets of 90 Irish multiplex schizophrenia families and follow-up of selected regions in all families provides evidence for multiple susceptibility genes
Molecular Psychiatry (2002)