Abstract
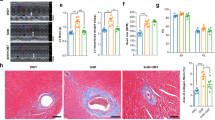
Elevated superoxide formation in cardiac extracts of apolipoprotein E–knockout (apoE-KO) mice has been reported. In addition, we previously reported that hypoxia increased oxidative stress in the aortas of apoE-KO mice, although we did not examine the effect of hypoxia on the heart. The aim of this study was to investigate the effect of chronic hypoxia on the left ventricular (LV) remodeling in apoE-KO mice treated with or without an angiotensin II receptor blocker. Male apoE-KO mice (n=83) and wild-type mice (n=34) at 15 weeks of age were kept under hypoxic conditions (oxygen, 10.0±0.5%) and treated with olmesartan (3 mg/kg/day) or vehicle for 3 weeks. Although LV pressure was not changed, hypoxia caused hypertrophy of cardiomyocytes and increased interstitial fibrosis in the LV myocardium. Furthermore, nuclear factor-κB (NF-κB) and matrix metalloproteinase (MMP)-9 activities were increased in apoE-KO mice exposed to chronic hypoxia. Olmesartan effectively suppressed the 4-hydroxy-2-nonenal protein expression and NF-κB and MMP-9 activities, and preserved the fine structure of the LV myocardium without affecting the LV pressure. In conclusion, olmesartan reduced oxidative stress, and attenuated the hypoxia-induced LV remodeling, in part through the inhibition of NF-κB and MMP-9 activities, in apoE-KO mice.
Similar content being viewed by others
Article PDF
References
Engstrom G, Lind P, Hedblad B, et al: Lung function and cardiovascular risk: relationship with inflammation-sensitive plasma proteins. Circulation 2002; 106: 2555–2560.
Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B : Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92: 78–84.
Nakashima H, Katayama T, Takagi C, et al: Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur Heart J 2006; 27: 2317–2322.
Alchanatis M, Tourkohoriti G, Kosmas EN, et al: Evidence for left ventricular dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J 2002; 20: 1239–1245.
Pfeffer MA, Braunwald E : Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation 1990; 81: 1161–1172.
Chin K, Shimizu K, Nakamura T, et al: Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation 1999; 100: 706–712.
Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR : Circulating cardiovascular risk factors in obstructive sleep apnea: data from randomized controlled trials. Thorax 2004; 59: 777–783.
Li J, Thorne LN, Punjabi NM, et al: Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 2005; 97: 698–706.
Kadotani H, Kadotani T, Young, et al: Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA 2001; 285: 2888–2890.
Gottli DJ, DeStefano AL, Foley DJ, et al: APOE ε4 is associated with obstructive sleep apnea/hypopnea. The Sleep Heart Health Study. Neurology 2004; 63: 664–668.
Saarelainen S, Lehtimaki T, Kallonen E, et al: No relation between apolipoprotein E alleles and obstructive sleep apnea. Clin Genet 1998; 53: 147–148.
Palmer LJ, Buxbaum SG, Larkin E, et al: A whole-genome scan for obstructive sleep apnea and obesity. Am J Hum Genet 2003; 72: 340–350.
Nakano D, Hayashi T, Tazawa N, et al: Chronic hypoxia accelerates the progression of atherosclerosis in apolipoprotein E–knockout mice. Hypertens Res 2005; 28: 837–845.
Wu JH, Hagaman J, Kim S, et al: Aortic constriction exacerbates atherosclerosis and induces cardiac dysfunction in mice lacking apolipoprotein E. Arterioscler Thromb Vasc Biol 2002; 22: 469–475.
Hartley CJ, Reddy AK, Madala S, et al: Hemodynamic changes in apolipoprotein E–knockout mice. Am J Physiol 2000; 279: H2326–H2334.
Godecke A, Ziegler M, Ding Z, Schrader J : Endothelial dysfunction of coronary resistance vessels in apoE−/− mice involves NO but not prostacyclin-dependent mechanisms. Cardiovasc Res 2002; 53: 253–262.
Kinugawa S, Tsutsui H, Hayashidani S, et al: Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 2000; 87: 392–398.
Kawamura N, Kubota T, Kawano S, et al: Blockade of NF-κB improves cardiac function and survival without affecting inflammation in TNF-α−induced cardiomyopathy. Cardiovasc Res 2005; 66: 520–529.
Gupta S, Young D, Sen S : Inhibition of NF-κB induces regression of cardiac hypertrophy, independent of blood pressure control, in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 2005; 289: H20–H29.
Li Y, Ha T, Gao X, et al: NF-κB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 2004; 287: H1712–H1720.
Harada K, Sugaya T, Murakami K, Yazaki Y, Komuro I : Angiotensin II type 1A receptor knockout mice display less left ventricular remodeling and improved survival after myocardial infarction. Circulation 1999; 100: 2093–2099.
Inamoto S, Hayashi T, Tazawa N, et al: Angiotensin-II receptor blocker exerts cardioprotection in diabetic rats exposed to hypoxia. Circ J 2006; 70: 787–792.
Mori T, Hayashi T, Sohmiya K, et al: Mechanisms of combined treatment with celiprolol and candesartan for ventricular remodeling in experimental heart failure. Circ J 2005; 69: 596–602.
Hayashi T, James TN, Buckingham DC : Ultrastructure and immunohistochemistry of the coronary chemoreceptor in human and canine hearts. Am Heart J 1995; 129: 946–959.
Matsuo T, Mori H, Nishimura Y, et al: Quantification of immunohistochemistry using an image analyser: correlation with hormone concentrations in pituitary adenomas. Histochem J 1995; 27: 989–996.
Okuda N, Hayashi T, Mori T, et al: Nifedipine enhances the cardioprotective effect of an angiotensin-II receptor blocker in an experimental animal model of heart failure. Hypertens Res 2005; 28: 431–438.
Rahman M, Nishiyama A, Guo P, et al: Effects of adrenomedullin on cardiac oxidative stress and collagen accumulation in aldosterone-dependent malignant hypertensive rats. J Pharmacol Exp Ther 2006; 318: 1323–1329.
Hayashi T, Nozawa M, Sohmiya K, et al: Efficacy of pancreatic transplantation on cardiovascular alterations in diabetic rats: an ultrastructural and immunohistochemical study. Transplant Proc 1998; 30: 335–338.
Shiina K, Tomiyama H, Takata Y, et al: Concurrent presence of metabolic syndrome in obstructive sleep apnea syndrome exacerbates the cardiovascular risk: a sleep clinic cohort study. Hypertens Res 2006; 29: 433–441.
Tanigawa T, Tachibana N, Yamagishi K, et al: Relationship between sleep-disordered breathing and blood pressure levels in community-based samples of Japanese men. Hypertens Res 2004; 27: 479–484.
McCarthy JJ, Parker A, Salem R, et al: Large scale association analysis for identification of genes underlying premature coronary heart disease: cumulative perspective from analysis of 111 candidate genes. J Med Genet 2004; 41: 334–341.
Kurian KC, Rai P, Sankaran S, Jacob B, Chiong J, Miller AB : The effect of statins in heart failure: beyond its cholesterol-lowering effect. J Cardiac Failure 2006; 12: 473–478.
Liu HW, Iwai M, Takeda-Matsubara Y, et al: Effect of estrogen and AT1 receptor blocker on neointima formation. Hypertension 2002; 40: 451–457.
Jinno T, Iwai M, Li Z, et al: Calcium channel blocker azelnidipine enhances vascular protective effects of AT1 receptor blocker olmesartan. Hypertension 2004; 43: 263–269.
Myslinski W, Dybala A, Mosiewicz J, Prystupa A, Hanzlik J : Cardiovascular abnormalities in patients with obstructive sleep apnoea syndrome. Wiad Lek 2005; 58: 78–83.
Kumar R, Pasha O, Khan AP, Gupta V : Renin angiotensin aldosterone system and ACE I/D gene polymorphism in high-altitude pulmonary edema. Aviat Space Environ Med 2004: 75: 981–983.
Chen L, Einbinder E, Zhang O, et al: Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 2005; 172: 915–920.
Adamy C, Oliviero P, Eddahibi S, et al: Cardiac modulations of ANG II receptor expression in rats with hypoxic pulmonary hypertension. Am J Physiol Heart Circ Physiol 2002; 283: H733–H740.
Zakheim RM, Molteni A, Mattioli L, Park M : Plasma angiotensin II levels in hypoxic and hypovolemic stress in unanesthetized rabbits. J Appl Physiol 1976; 41: 462–465.
El-Sokkary GH, Khidr BM, Younes HA : Role of melatonin in reducing hypoxia-induced oxidative stress and morphological changes in the liver of male mice. Eur J Pharmacol 2006; 540: 107–114.
Daugherty A, Manning MW, Cassis LA : Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E–deficient mice. J Clin Invest 2000; 105: 1605–1612.
Fletcher EC, Orolinova N, Bader M : Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol 2002; 92: 627–633.
Greenberg H, Ye X, Wilson D, et al: Chronic intermittent hypoxia activates nuclear factor-κB in cardiovascular tissues in vivo. Biochem Biophys Res Commun 2006; 343: 591–596.
Thomas CV, Coker ML, Zellner JL, et al: Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation 1998; 97: 1708–1715.
Reinhardt D, Sigusch HH, Henbe J, et al: Cardiac remodelling in end stage heart failure: upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart 2002; 88: 525–530.
Vellaichamy E, Khurana ML, Fink J, Pandey KN : Involvement of the NF-κB/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A. J Biol Chem 2005; 280: 19230–19242.
Sakata Y, Yamamoto K, Mano T, et al: Activation of matrix metalloproteinases precedes left ventricular remodeling in hypertensive heart failure rats: its inhibition as a primary effect of angiotensin-converting enzyme inhibitor. Circulation 2004; 109: 2143–2149.
Li YY, McTiernan CF, Feldman AM : Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res 2000; 46: 214–224.
Maquart FX, Bellon G, Chaqour B, et al: In vivo stimulation of connective tissue accumulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ in rat experimental wounds. J Clin Invest 1993; 92: 2368–2376.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, C., Hayashi, T., Mori, T. et al. Angiotensin II Receptor Blocker Reduces Oxidative Stress and Attenuates Hypoxia-Induced Left Ventricular Remodeling in Apolipoprotein E–Knockout Mice. Hypertens Res 30, 1219–1230 (2007). https://doi.org/10.1291/hypres.30.1219
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1291/hypres.30.1219
Keywords
This article is cited by
-
Augmented O-GlcNAcylation exacerbates right ventricular dysfunction and remodeling via enhancement of hypertrophy, mitophagy, and fibrosis in mice exposed to long-term intermittent hypoxia
Hypertension Research (2023)
-
Effect of low-dose hydrocortisone and inhaled nitric oxide on inflammatory mediators and local pulmonary metalloproteinases activity in LPS-induced sepsis in piglets
Scientific Reports (2023)
-
Augmented O-GlcNAcylation attenuates intermittent hypoxia-induced cardiac remodeling through the suppression of NFAT and NF-κB activities in mice
Hypertension Research (2019)
-
Olmesartan reduces inflammatory biomarkers in patients with stable coronary artery disease undergoing percutaneous coronary intervention: results from the OLIVUS trial
Heart and Vessels (2014)
-
Celiprolol reduces oxidative stress and attenuates left ventricular remodeling induced by hypoxic stress in mice
Hypertension Research (2013)