Abstract
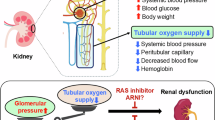
Recent studies emphasize the role of chronic hypoxia in the kidney as a final common pathway to end-stage renal failure (ESRD). Hypoxia of tubular cells leads to apoptosis or epithelial-mesenchymal transdifferentiation, which in turn exacerbates the fibrosis of the kidney with the loss of peritubular capillaries and subsequent chronic hypoxia, setting in train a vicious cycle whose end-point is ESRD. While fibrotic kidneys in an advanced stage of renal disease are devoid of peritubular capillary blood supply and oxygenation to the corresponding region, imbalances in vasoactive substances can cause chronic hypoxia even in the early phase of kidney disease. Among various vasoactive substances, local activation of the renin-angiotensin system (RAS) is particularly important because it can lead to the constriction of efferent arterioles, hypoperfusion of postglomerular peritubular capillaries, and subsequent hypoxia of the tubulointerstitium in the downstream compartment. In addition, angiotensin II induces oxidative stress via the activation of NADPH oxidase. Oxidative stress damages endothelial cells directly, causing the loss of peritubular capillaries, and also results in relative hypoxia due to inefficient cellular respiration. Thus, angiotensin II induces renal hypoxia via both hemodynamic and nonhemodynamic mechanisms. In the past two decades, considerable gains have been realized in retarding the progression of chronic kidney disease by emphasizing blood pressure control and blockade of the RAS. Chronic hypoxia in the kidney is an ideal therapeutic target, and the beneficial effects of blockade of RAS in kidney disease are, at least in part, mediated by the amelioration of local hypoxia.
Similar content being viewed by others
Article PDF
References
Kiberd B : The chronic kidney disease epidemic: stepping back and looking forward. J Am Soc Nephrol 2006; 17: 2967–2973.
Sarnak MJ, Levey AS, Schoolwerth AC, et al: Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003; 42: 1050–1065.
Brosius FC 3rd, Hostetter TH, Kelepouris E, et al: Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease: a science advisory from the American Heart Association Kidney and Cardiovascular Disease Council; the Councils on High Blood Pressure Research, Cardiovascular Disease in the Young, and Epidemiology and Prevention; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: Developed in Collaboration with the National Kidney Foundation. Hypertension 2006; 48: 751–755.
Nangaku M, Fujita T : Chronic interstitial nephritis, in Johnson RJ, Feehally J, Floege J ( eds): Comprehensive Clinical Nephrology, 3rd ed. Philadelphia, Elsevier, 2007, pp 703–716.
Flack JM, Peters R, Shafi T, Alrefai H, Nasser SA, Crook E : Prevention of hypertension and its complications: theoretical basis and guidelines for treatment. J Am Soc Nephrol 2003; 14 ( Suppl 2): S92–S98.
Levey AS : Nondiabetic kidney disease. N Engl J Med 2002; 347: 1505–1511.
Bakris GL, Weir MR : Angiotensin-converting enzyme inhibitor associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med 2000; 160: 685–693.
Maki DD, Ma JZ, Louis TA, Kasiske BL : Long-term effects of antihypertensive agents on proteinuria and renal function. Arch Intern Med 1995; 155: 1073–1080.
Kasiske BL, Kalil RS, Ma JZ, Liao M, Keane WF : Effect of antihypertensive therapy on the kidney in patients with diabetes: a meta-regression analysis. Ann Intern Med 1993; 118: 129–138.
Jafar TH, Schmid CH, Landa M, et al: Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135: 73–87.
Nangaku M, Ohse T, Tanaka T, Kojima I, Fujita T : Renoprotection with anti-hypertensives: reduction of proteinuria and improvement of oxygenation via inhibition of the renin-angiotensin system. Curr Hypertens Rev 2005; 1: 67–76.
Weinberg MS, Weinberg AJ, Cord R, Zappe DH : The effect of high dose angiotensin II receptor blockade beyond maximal recommended doses in reducing urinary protein excretion. J Renin Angiotensin Aldosterone Syst 2001; 2 ( Suppl 1): S196–S198.
Nangaku M : Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 2006; 17: 17–25.
Norman JT, Fine LG : Intrarenal oxygenation in chronic renal failure. Clin Exp Pharmacol Physiol 2006; 33: 989–996.
Eckardt KU, Bernhardt WM, Weidemann A, et al: Role of hypoxia in the pathogenesis of renal disease. Kidney Int 2005; 99: S46–S51.
Nakagawa T, Kang DH, Ohashi R, et al: Tubulointerstitial disease: role of ischemia and microvascular disease. Curr Opin Nephrol Hypertens 2003; 12: 233–241.
Nangaku M : Hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. Nephron Exp Nephrol 2004; 98: e8–e12.
Nangaku M : Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med 2004; 43: 9–17.
Ohashi R, Shimizu A, Masuda Y, et al: Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol 2002; 13: 1795–1805.
Ohashi R, Kitamura H, Yamanaka N : Peritubular capillary injury during the progression of experimental glomerulonephritis in rats. J Am Soc Nephrol 2000; 11: 47–56.
Kang DH, Anderson S, Kim YG, et al: Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis 2001; 37: 601–611.
Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ : Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 2001; 12: 1448–1457.
Kairaitis LK, Wang Y, Gassmann M, Tay YC, Harris DC : HIF-1alpha expression follows microvascular loss in advanced murine adriamycin nephrosis. Am J Physiol Renal Physiol 2005; 288: F198–F206.
Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS : Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol 2003; 163: 2289–2301.
Sun D, Feng J, Dai C, et al: Role of peritubular capillary loss and hypoxia in progressive tubulointerstitial fibrosis in a rat model of aristolochic acid nephropathy. Am J Nephrol 2006; 26: 363–371.
Zhang B, Liang X, Shi W, et al: Role of impaired peritubular capillary and hypoxia in progressive interstitial fibrosis after 56 subtotal nephrectomy of rats. Nephrology 2005; 10: 351–357.
Matsumoto M, Tanaka T, Yamamoto T, et al: Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J Am Soc Nephrol 2004; 15: 1574–1581.
Izuhara Y, Nangaku M, Inagi R, et al: Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. J Am Soc Nephrol 2005; 16: 3631–3641.
Ries M, Basseau F, Tyndal B, et al: Renal diffusion and BOLD MRI in experimental diabetic nephropathy. J Magn Reson Imaging 2003; 17: 104–113.
Tanaka T, Miyata T, Inagi R, Fujita T, Nangaku M : Hypoxia in renal disease with proteinuria and/or glomerular hypertension. Am J Pathol 2004; 165: 1979–1992.
Tanaka T, Kato H, Kojima I, et al: Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J Gerontol A Biol Sci Med Sci 2006; 61: 795–805.
Safran M, Kim WY, O'Connell F, et al: Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci U S A 2006; 103: 105–110.
Lazarus JM, Hampers CL, Merrill JP : Hypertension in chronic renal failure: treatment with hemodialysis and nephrectomy. Arch Intern Med 1974; 133: 1059–1065.
Goldblatt H, Lynch J, Hanzal RF, Summerville WW : Studies on experimental hypertension: Part I: the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 1934; 59: 347–379.
Oparil S, Haber E : The renin-angiotensin system. N Engl J Med 1974; 291: 389–401.
Kobori H, Nangaku M, Navar LG, Nishiyama A : Independent regulation of intrarenal angiotensin II and impact of antihypertensive agents. Pharmacol Rev 2007; 59: 251–287.
Takase O, Marumo T, Imai N, et al: NF-kappaB–dependent increase in intrarenal angiotensin II induced by proteinuria. Kidney Int 2005; 68: 464–473.
Wolf G : Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int 2006; 70: 1914–1919.
Shao J, Nangaku M, Miyata T, et al: Imbalance of T-cell subsets in angiotensin II–infused hypertensive rats with kidney injury. Hypertension 2003; 42: 31–38.
Kitayama H, Maeshima Y, Takazawa Y, et al: Regulation of angiogenic factors in angiotensin II infusion model in association with tubulointerstitial injuries. Am J Hypertens 2006; 19: 718–727.
Manotham K, Tanaka T, Matsumoto M, et al: Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int 2004; 65: 871–880.
Tanaka T, Nangaku M, Miyata T, et al: Blockade of calcium influx through L-type calcium channels attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells. J Am Soc Nephrol 2004; 15: 2320–2333.
Tanaka T, Miyata T, Inagi R, et al: Hypoxia-induced apoptosis in cultured glomerular endothelial cells—involvement of mitochondrial pathways. Kidney Int 2003; 64: 2020–2032.
Tanaka T, Hanafusa N, Ingelfinger JR, Ohse T, Fujita T, Nangaku M : Hypoxia induces apoptosis in SV40-immortalized rat proximal tubular cells through the mitochondrial pathways, devoid of HIF-1–mediated upregulation of Bax. Biochem Biophys Res Commun 2003; 309: 222–231.
Kondo N, Kiyomoto H, Yamamoto T, et al: Effects of calcium channel blockade on angiotensin II–induced peritubular ischemia in rats. J Pharmacol Exp Ther 2006; 316: 1047–1052.
Schachinger H, Klarhofer M, Linder L, Drewe J, Scheffler K : Angiotensin II decreases the renal MRI blood oxygenation level–dependent signal. Hypertension 2006; 47: 1062–1066.
Ando K, Fujita T : Role of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in the development of hypertensive organ damage. Clin Exp Nephrol 2004; 8: 178–182.
Nagase M, Ando K, Nagase T, Kaname S, Sawamura T, Fujita T : Redox-sensitive regulation of LOX-1 gene expression in vascular endothelium. Biochem Biophys Res Commun 2001; 281: 720–725.
Ueno T, Kaname S, Takaichi K, et al: LOX-1, an oxidized low-density lipoprotein receptor, was upregulated in the kidneys of chronic renal failure rats. Hypertens Res 2003; 26: 117–122.
Nagase M, Kaname S, Nagase T, et al: Expression of LOX-1, an oxidized low-density lipoprotein receptor, in experimental hypertensive glomerulosclerosis. J Am Soc Nephrol 2000; 11: 1826–1836.
Laycock SK, Vogel T, Forfia PR, et al: Role of nitric oxide in the control of renal oxygen consumption and the regulation of chemical work in the kidney. Circ Res 1998; 82: 1263–1271.
Deng A, Miracle CM, Suarez JM, et al: Oxygen consumption in the kidney: effects of nitric oxide synthase isoforms and angiotensin II. Kidney Int 2005; 68: 723–730.
Adler S, Huang H, Loke KE, et al: Endothelial nitric oxide synthase plays an essential role in regulation of renal oxygen consumption by NO. Am J Physiol Renal Physiol 2001; 280: F838–F843.
Welch WJ, Mendonca M, Aslam S, Wilcox CS : Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2K,1C kidney. Hypertension 2003; 41: 692–696.
Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS : Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol 2005; 288: H22–H28.
Adler S, Huang H : Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol 2004; 287: F907–F913.
Abramov AY, Scorziello A, Duchen MR : Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 2007; 27: 1129–1138.
Shimosawa T, Fujita T : Adrenomedullin and its related peptide. Endocr J 2005; 52: 1–10.
Ando K, Shimosawa T, Fujita T : Adrenomedullin in vascular diseases. Curr Hypertens Rep 2004; 6: 55–59.
Liu J, Shimosawa T, Matsui H, et al: Adrenomedullin inhibits angiotensin II–induced oxidative stress via Csk-mediated inhibition of Src activity. Am J Physiol Heart Circ Physiol 2007; 292: H1714–H1721.
Matsui H, Shimosawa T, Itakura K, Guanqun X, Ando K, Fujita T : Adrenomedullin can protect against pulmonary vascular remodeling induced by hypoxia. Circulation 2004; 109: 2246–2251.
Shankland SJ : The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 2006; 69: 2131–2147.
Kriz W, Lemley KV : The role of the podocyte in glomerulosclerosis. Curr Opin Nephrol Hypertens 1999; 8: 489–497.
Racusen LC, Prozialeck DH, Solez K : Glomerular epithelial cell changes after ischemia or dehydration. Possible role of angiotensin II. Am J Pathol 1984; 114: 157–163.
Liebau MC, Lang D, Bohm J, et al: Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol 2006; 290: F710–F719.
Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N : Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 2004; 15: 1475–1487.
Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T : Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 2007; 49: 355–364.
Nagase M, Yoshida S, Shibata S, et al: Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol 2006; 17: 3438–3446.
Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T : Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 2006; 47: 1084–1093.
Kojima I, Tanaka T, Inagi R, et al: Protective role of HIF-2 alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol 2007; 18: 1218–1226.
Manotham K, Tanaka T, Ohse T, et al: A biological role of HIF-1 in the renal medulla. Kidney Int 2005; 67: 1428–1439.
Rajagopalan S, Olin J, Deitcher S, et al: Use of a constitutively active hypoxia-inducible factor-1alpha transgene as a therapeutic strategy in no-option critical limb ischemia patients: phase I dose-escalation experience. Circulation 2007; 115: 1234–1243.
Tanaka T, Matsumoto M, Inagi R, et al: Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney Int 2005; 68: 2714–2725.
Tanaka T, Kojima I, Ohse T, et al: Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest 2005; 85: 292–1307.
Tanaka T, Kojima I, Ohse T, et al: Hypoxia-inducible factor modulates tubular cell survival in cisplatin nephrotoxicity. Am J Physiol Renal Physiol 2005; 289: F1123–F1133.
Kudo Y, Kakinuma Y, Mori Y, et al: Hypoxia-inducible factor-1alpha is involved in the attenuation of experimentally induced rat glomerulonephritis. Nephron Exp Nephrol 2005; 100: e95–e103.
Matsumoto M, Makino Y, Tanaka T, et al: Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol 2003; 14: 1825–1832.
Bernhardt WM, Campean V, Kany S, et al: Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 2006; 17: 1970–1978.
Norman JT, Stidwill R, Singer M, Fine LG : Angiotensin II blockade augments renal cortical microvascular pO2 indicating a novel, potentially renoprotective action. Nephron Physiol 2003; 94: 39–46.
Manotham K, Tanaka T, Matsumoto M, et al: Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol 2004; 15: 1277–1288.
Miyata T, van Ypersele de Strihou C, Ueda Y, et al: Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol 2002; 13: 2478–2487.
Shao J, Nangaku M, Inagi R, et al: Receptor-independent intracellular radical scavenging activity of an angiotensin II receptor blocker. J Hypertens 2007; 25: 1643–1649.
Welch WJ, Baumgartl H, Lubbers D, Wilcox CS : Renal oxygenation defects in the spontaneously hypertensive rat: role of AT1 receptors. Kidney Int 2003; 63: 202–208.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nangaku, M., Fujita, T. Activation of the Renin-Angiotensin System and Chronic Hypoxia of the Kidney. Hypertens Res 31, 175–184 (2008). https://doi.org/10.1291/hypres.31.175
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1291/hypres.31.175
Keywords
This article is cited by
-
Renin-angiotensin system activation: may it increase frequency of obstructive sleep apnea in patients with autosomal dominant polycystic kidney disease?
Sleep and Breathing (2023)
-
Association between chronic kidney disease and open-angle glaucoma in South Korea: a 12-year nationwide retrospective cohort study
Scientific Reports (2022)
-
Renoprotective effects of the novel prostaglandin EP4 receptor-selective antagonist ASP7657 in 5/6 nephrectomized chronic kidney disease rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2019)
-
Pathological cardiac remodeling occurs early in CKD mice from unilateral urinary obstruction, and is attenuated by Enalapril
Scientific Reports (2018)
-
Regulation, signalling and functions of hormonal peptides in pulmonary vascular remodelling during hypoxia
Endocrine (2018)