Abstract
Objective:
To develop a non-invasive method of studying brain mechanisms involved in energy homeostasis and appetite regulation in humans by using visual food cues that are relevant to individuals attempting weight loss.
Design:
Functional magnetic resonance imaging (fMRI) was used to compare brain activation in regions of interest between groups of food photographs.
Participants:
Ten healthy, non-obese women who were not dieting for weight loss.
Measurements:
Independent raters viewed food photographs and evaluated whether the foods depicted should be eaten by individuals attempting a calorically-restricted diet. Based on their responses, we categorized photographs into ‘non-fattening’ and ‘fattening’ food groups, the latter characterized by high-caloric content and usually also high-fat or high-sugar content. Blood oxygen level-dependent (BOLD) response was measured by fMRI while participants viewed photographs of ‘fattening’ food, ‘non-fattening’ food, and non-food objects.
Results:
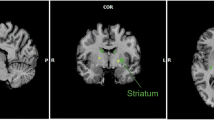
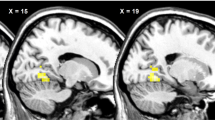
Viewing photographs of fattening food compared with non-food objects resulted in significantly greater activation in the brainstem; hypothalamus; left amygdala; left dorsolateral prefrontal cortex; left orbitofrontal cortex; right insular cortex; bilateral striatum, including the nucleus accumbens, caudate nucleus, and putamen; bilateral thalamus; and occipital lobe. By comparison, only the occipital region had greater activation by non-fattening food than by object photographs. Combining responses to all food types resulted in attenuation of activation in the brainstem, hypothalamus, and striatum.
Conclusion:
These findings suggest that, in non-obese women, neural circuits engaged in energy homeostasis and reward processing are selectively attuned to representations of high-calorie foods that are perceived as fattening. Studies to investigate hormonal action or manipulation of energy balance may benefit from fMRI protocols that contrast energy-rich food stimuli with non-food or low-calorie food stimuli.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW . Central nervous system control of food intake and body weight. Nature 2006; 443: 289–295.
Kelley AE, Baldo BA, Pratt WE, Will MJ . Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 2005; 86: 773–795.
Stice E, Spoor S, Bohon C, Small DM . Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008; 322: 449–452.
Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 2004; 21: 1790–1797.
Cheng Y, Meltzoff AN, Decety J . Motivation modulates the activity of the human mirror-neuron system. Cereb Cortex 2007; 17: 1979–1986.
Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR . Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr 2007; 86: 965–971.
Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC . Leptin regulates striatal regions and human eating behavior. Science 2007; 317: 1355.
Fuhrer D, Zysset S, Stumvoll M . Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity 2008; 16: 945–950.
Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS et al. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage 2005; 27: 669–676.
Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA . Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage 2003; 19: 1381–1394.
Simmons WK, Martin A, Barsalou LW . Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex 2005; 15: 1602–1608.
Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007; 37: 410–421.
LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM . Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci 2001; 115: 493–500.
Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA 2007; 104: 18276–18279.
Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE . Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008; 41: 636–647.
Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J . Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 2008; 118: 2583–2591.
Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC . Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res 2006; 169: 111–119.
Afari N, Noonan C, Goldberg J, Edwards K, Gadepalli K, Osterman B et al. University of Washington Twin Registry: construction and characteristics of a community-based twin registry. Twin Res Hum Genet 2006; 9: 1023–1029.
Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG . Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 2003; 964: 107–115.
Naleid AM, Grace MK, Cummings DE, Levine AS . Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 2005; 26: 2274–2279.
Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N . Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage 2006; 32: 1273–1280.
Rolls ET . Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav 2005; 85: 45–56.
Del Parigi A, Chen K, Gautier JF, Salbe AD, Pratley RE, Ravussin E et al. Sex differences in the human brain's response to hunger and satiation. Am J Clin Nutr 2002; 75: 1017–1022.
Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA 2009; 106: 1249–1254.
DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007; 31: 440–448.
Sun T, Walsh CA . Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci 2006; 7: 655–662.
Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE . Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage 1997; 6: 156–167.
Del Parigi A, Gautier JF, Chen K, Salbe AD, Ravussin E, Reiman E et al. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci 2002; 967: 389–397.
Ahima RS, Antwi DA . Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am 2008; 37: 811–823.
Logothetis N . What we can and cannot do with fMRI. Nature 2008; 453: 869–878.
Acknowledgements
This work was supported by NIH grant K23 DK070826-01 (PI: Schur). We also acknowledge the donation of a number of photographs used for this study by Great American Stock (Brookfield, WI, USA). We thank our participants for their contribution and the twins in the University of Washington Twin Registry for their dedication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schur, E., Kleinhans, N., Goldberg, J. et al. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes 33, 653–661 (2009). https://doi.org/10.1038/ijo.2009.56
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ijo.2009.56
Keywords
This article is cited by
-
Food stimuli decrease activation in regions of the prefrontal cortex related to executive function: an fNIRS study
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2023)
-
Disruption of orbitofrontal-hypothalamic projections in a murine ALS model and in human patients
Translational Neurodegeneration (2021)
-
The interrelationship of body mass index with gray matter volume and resting-state functional connectivity of the hypothalamus
International Journal of Obesity (2020)
-
Brain activation during repeated imagining of chocolate consumption: a functional magnetic resonance imaging study
Hormones (2018)
-
A Novel Spatiotemporal Longitudinal Methodology for Predicting Obesity Using Near Infrared Spectroscopy (NIRS) Cerebral Functional Activity Data
Cognitive Computation (2018)