Abstract
Background:
Although obesity increases the risk of developing cardiomyopathy, the mechanisms underlying the development of this cardiomyopathy are incompletely understood. As obesity is also associated with increased intramyocardial triacylglycerol (TAG) deposition, also referred to as cardiac steatosis, we hypothesized that alterations in myocardial TAG metabolism and excess TAG accumulation contribute to obesity-induced cardiomyopathy.
Objective and design:
To test if increased TAG catabolism could ameliorate obesity-induced cardiac steatosis and dysfunction, we utilized wild-type (WT) mice and mice with cardiomyocyte-specific overexpression of adipose triglyceride lipase (MHC-ATGL mice), which regulates cardiac TAG hydrolysis. WT and MHC-ATGL mice were fed either regular chow (13.5 kcal% fat) or high fat–high sucrose (HFHS; 45 kcal% fat and 17 kcal% sucrose) diet for 16 weeks to induce obesity and mice were subsequently studied at the physiological, biochemical and molecular level.
Results:
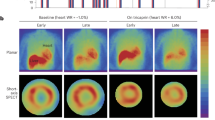
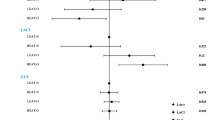
Obese MHC-ATGL mice were protected from increased intramyocardial TAG accumulation, despite similar increases in body weight and systemic insulin resistance as obese WT mice. Importantly, analysis of in vivo cardiac function using transthoracic echocardiography showed that ATGL overexpression protected from obesity-induced systolic and diastolic dysfunction and ventricular dilatation. Ex vivo working heart perfusions revealed impaired cardiac glucose oxidation following obesity in both WT and MHC-ATGL mice, which was consistent with similar impaired cardiac insulin signaling between genotypes. However, hearts from obese MHC-ATGL mice exhibited reduced reliance on palmitate oxidation when compared with the obese WT, which was accompanied by decreased expression of proteins involved in fatty acid uptake, storage and oxidation in MHC-ATGL hearts.
Conclusion:
These findings suggest that cardiomyocyte-specific ATGL overexpression was sufficient to prevent cardiac steatosis and decrease fatty acid utilization following HFHS diet feeding, leading to protection against obesity-induced cardiac dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG et al. Obesity and the risk of heart failure. N Engl J Med 2002; 347: 305–313.
Harmancey R, Wilson CR, Taegtmeyer H . Adaptation and maladaptation of the heart in obesity. Hypertension 2008; 52: 181–187.
Abel ED, Litwin SE, Sweeney G . Cardiac remodeling in obesity. Physiol Rev 2008; 88: 389–419.
Alpert MA . Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci 2001; 321: 225–236.
Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 2004; 18: 1692–1700.
Szczepaniak LS, Victor RG, Orci L, Unger RH . Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res 2007; 101: 759–767.
Utz W, Engeli S, Haufe S, Kast P, Hermsdorf M, Wiesner S et al. Myocardial steatosis, cardiac remodelling and fitness in insulin-sensitive and insulin-resistant obese women. Heart 2011; 97: 1585–1589.
Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D'Ambrosia G, Arbique D, Vongpatanasin W et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med 2003; 49: 417–423.
McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007; 116: 1170–1175.
Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008; 52: 1793–1799.
Korosoglou G, Humpert PM, Ahrens J, Oikonomou D, Osman NF, Gitsioudis G et al. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magnet Resonan Imag 2012; 35: 804–811.
Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 2003; 144: 3483–3490.
Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 2004; 10: 1245–1250.
Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 2001; 107: 813–822.
Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 2002; 109: 121–130.
Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 2000; 97: 1784–1789.
Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S et al. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes 2002; 51: 2587–2595.
Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S et al. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest 2010; 120: 3443–3454.
Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem 2009; 284: 36312–36323.
Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A et al. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res 2007; 100: 1208–1217.
Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest 2003; 111: 419–426.
Ge F, Hu C, Hyodo E, Arai K, Zhou S, Lobdell IvH et al. Cardiomyocyte triglyceride accumulation and reduced ventricular function in mice with obesity reflect increased long chain fatty acid uptake and de novo Fatty Acid synthesis. J Obes 2012; 2012: 205648.
Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006; 312: 734–737.
Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G . Triglyceride deposit cardiomyovasculopathy. N Engl J Med 2008; 359: 2396–2398.
Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R . Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab 2009; 297: E289–E296.
Kienesberger PC, Pulinilkunnil T, Sung MM, Nagendran J, Haemmerle G, Kershaw EE et al. Myocardial ATGL overexpression decreases the reliance on fatty acid oxidation and protects against pressure overload-induced cardiac dysfunction. Mol Cell Biol 2012; 32: 740–750.
Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab 2010; 12: 533–544.
Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med 2011; 17: 1076–1085.
Bodart V, Febbraio M, Demers A, McNicoll N, Pohankova P, Perreault A et al. CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circ Res 2002; 90: 844–849.
Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C et al. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem 2010; 285: 2918–2929.
Pulinilkunnil T, Kienesberger PC, Nagendran J, Waller TJ, Young ME, Kershaw EE et al. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes 2013; 62: 1464–1477.
Layland J, Solaro RJ, Shah AM . Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 2005; 66: 12–21.
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999; 98: 115–124.
Lee J, Xu Y, Lu L, Bergman B, Leitner JW, Greyson C et al. Multiple abnormalities of myocardial insulin signaling in a porcine model of diet-induced obesity. Am J Physiol Heart Circ Physiol 2010; 298: H310–H319.
Zhang Y, Yuan M, Bradley KM, Dong F, Anversa P, Ren J . Insulin-like growth factor 1 alleviates high-fat diet-induced myocardial contractile dysfunction: role of insulin signaling and mitochondrial function. Hypertension 2012; 59: 680–693.
Rider OJ, Cox P, Tyler D, Clarke K, Neubauer S . Myocardial substrate metabolism in obesity. Int J Obes (Lond) 2013; 37: 972–979.
Kienesberger PC, Pulinilkunnil T, Nagendran J, Dyck JR . Myocardial triacylglycerol metabolism. J Mol Cell Cardiol 2013; 55: 101–110.
Liu L, Yu S, Khan RS, Ables GP, Bharadwaj KG, Hu Y et al. DGAT1 deficiency decreases PPAR expression and does not lead to lipotoxicity in cardiac and skeletal muscle. J Lipid Res 2011; 52: 732–744.
Khan RS, Drosatos K, Goldberg IJ . Creating and curing fatty hearts. Curr Opin Clin Nutr Metab Care 2010; 13: 145–149.
Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 2003; 100: 3077–3082.
Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 2005; 96: 225–233.
Kienesberger PC, Lee D, Pulinilkunnil T, Brenner DS, Cai L, Magnes C et al. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J Biol Chem 2009; 284: 30218–30229.
Badin PM, Louche K, Mairal A, Liebisch G, Schmitz G, Rustan AC et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes 2011; 60: 1734–1742.
Turpin SM, Hoy AJ, Brown RD, Rudaz CG, Honeyman J, Matzaris M et al. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia 2011; 54: 146–156.
Acknowledgements
This work was supported by grants from the Canadian Institute of Health Research and Canadian Diabetes Association to JRBD, post-doctoral fellowships from the Heart and Stroke Foundation of Canada and the Canadian Diabetes Association to PCK, and Alberta Innovates-Health Solutions (AIHS) post-doctoral fellowships to TP and PCK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pulinilkunnil, T., Kienesberger, P., Nagendran, J. et al. Cardiac-specific adipose triglyceride lipase overexpression protects from cardiac steatosis and dilated cardiomyopathy following diet-induced obesity. Int J Obes 38, 205–215 (2014). https://doi.org/10.1038/ijo.2013.103
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ijo.2013.103
Keywords
This article is cited by
-
Validation of optimal reference genes for quantitative real time PCR in muscle and adipose tissue for obesity and diabetes research
Scientific Reports (2017)
-
Comparative proteomics reveals abnormal binding of ATGL and dysferlin on lipid droplets from pressure overload-induced dysfunctional rat hearts
Scientific Reports (2016)
-
Targeting metabolic disturbance in the diabetic heart
Cardiovascular Diabetology (2015)